Abstract
Background
Neonatal intensive care unit (NICU) patients are at increased risk for autism spectrum disorder (ASD). Autonomic nervous system aberrancy has been described in children with ASD, and we aimed to identify heart rate (HR) patterns in NICU patients associated with eventual ASD diagnosis.
Methods
This retrospective cohort study included NICU patients from 2009 to 2016 with archived HR data and follow-up beyond age 3 years. Medical records provided clinical variables and ASD diagnosis. HR data were compared in infants with and without ASD.
Results
Of the 2371 patients, 88 had ASD, and 689,016 h of data were analyzed. HR skewness (HRskw) was significantly different between ASD and control infants. Preterm infants at early postmenstrual ages (PMAs) had negative HRskw reflecting decelerations, which increased with maturation. From 34 to 42 weeks PMA, positive HRskw toward accelerations was higher in males with ASD. In 931 males with at least 4 days of HR data, overall ASD prevalence was 5%, whereas 11% in the top 5th HRskw percentile had ASD.
Conclusion
High HRskw in NICU males, perhaps representing autonomic imbalance, was associated with increased ASD risk. Further study is needed to determine whether HR analysis identifies highest-risk infants who might benefit from earlier screening and therapies.
Impact
-
In a large retrospective single-center cohort of NICU patients, we found that high positive skewness of heart rate toward more accelerations was significantly associated with increased risk of eventual autism spectrum disorder diagnosis in male infants but not in females.
-
Existing literature describes differences in heart rate characteristics in children, adolescents, and adults with autism spectrum disorders, but the finding from our study in NICU infants is novel.
-
Heart rate analysis during the NICU stay might identify, among an inherently high-risk population, those infants with especially high risk of ASD who might benefit from earlier screening and therapies.
Similar content being viewed by others
Introduction
Infants requiring intensive care at birth are at increased risk of eventual diagnosis of autism spectrum disorder (ASD). While the overall incidence of ASD in the United States is estimated at 1.7%,1 preterm infants are about four times more likely than term infants to be diagnosed with ASD.2 A variety of conditions associated with preterm birth may contribute to ASD risk, including prenatal or neonatal inflammation.3 Fetal or neonatal hypoxia leading to white matter injury may also contribute to autism.4 In term infants, hypoxic ischemic encephalopathy and other conditions requiring intensive care at birth are known to be associated with increased risk for later diagnosis of ASD.5,6,7,8,9,10,11 Identifying neonatal intensive care unit (NICU) patients with the highest risk of ASD could facilitate earlier targeted interventions during critical periods of brain development, potentially translating to improved long-term outcomes.12,13,14
Heart rate (HR), which is continuously monitored in all NICU patients, might provide clues about risk for ASD. HR is regulated by the autonomic nervous system, with sympathetic and parasympathetic activation leading to accelerations and decelerations, respectively, in response to internal or external stimuli.15 Children with ASD have been reported to have differences in HR patterns compared to neurotypical individuals, including higher HR.16,17 The mechanism of these findings is not well understood, but there is some evidence of overactive sympathetic tone18,19,20 and decreased parasympathetic or vagal tone.21,22,23,24,25 These HR differences have particularly been noted during sleep and in response to social and non-social stimuli.26 In light of these findings in older individuals with ASD, we hypothesized that atypical HR patterns would be found in some NICU patients with eventual diagnosis of ASD.
Comprehensive analysis of HR requires specialized equipment and systems for collecting, storing, and analyzing bedside monitor data. The University of Virginia (UVa) NICU has had such a system in place for the past decade, enabling us to analyze cardiorespiratory patterns and develop algorithms to predict various adverse outcomes,27,28,29 including intracranial hemorrhage30 and hypoxic–ischemic brain injury.31 In the current study, we aimed to identify HR patterns of preterm and term NICU patients later diagnosed with ASD. Our specific hypothesis was that patterns involving high HR, perhaps representing sympathetic hyperarousal, might be identified in the neonatal period.
Methods
Patient population and clinical data collection
The retrospective study cohort was drawn from all infants admitted to the level IV NICU of UVa from 2009 to 2016. We included all former NICU patients with any archived bedside monitor HR data available from the NICU stay who received medical care in the UVa Health System beyond 3 years of age. Our general guideline is to schedule all high-risk infants for neurodevelopmental follow-up through at least 2 years of age, and beyond that if issues are identified. Diagnosis of ASD was confirmed by three authors with medical training (K.R.B., K.N.K., K.D.F.) reviewing the records of every child with an International Classification of Diseases (ICD)-9 or ICD-10 code associated with ASD. We additionally queried the electronic medical record data warehouse for the word “autism” in any notes. Most ASD diagnoses were made by a UVa neurodevelopmental and behavioral pediatrician based on Diagnostic and Statistical Manual of Mental Disorders criteria. Some ASD diagnoses were made by standardized testing such as the Autism Diagnostic Observation Schedule. Controls without ASD were those with no ICD diagnosis or no notes in the medical record confirming an ASD diagnosis. Children with other developmental and behavioral disabilities were not excluded from the control group.
Demographic and clinical data from all included infants were obtained from the NICU database (NeoData, Isoprime, Chicago, IL) and the electronic medical record. Maternal diabetes included Type I and Type II and gestational diabetes. Congenital cardiac malformation included those diagnosed on echocardiogram during the NICU stay, excluding atrial septal defect and patent ductus arteriosus. Diagnosis of brain injury included severe intraventricular hemorrhage (Grade III or IV), cystic periventricular leukomalacia, hypoxic–ischemic encephalopathy, cerebral infarct or thrombosis, and electroencephalogram-confirmed seizures. Diagnosis of brain malformation included congenital hydrocephalus and other malformations diagnosed on brain magnetic resonance imaging during the NICU stay.
This was an observational retrospective study and was approved by the UVa Institutional Review Board with waiver of consent.
HR data collection and analysis
All NICU bedside monitor vital sign data have been collected from all UVa NICU patients since 2009, using the BedMaster system (Excel Medical, Jupiter, FL). Electrocardiogram HR was sampled every 2 s (0.5 Hz). Values of zero were removed as incontrovertible artifact. HR characteristics (4 mathematical moments: mean, standard deviation, skewness, and kurtosis) were calculated in 10-min segments and averaged each hour.
Statistics
Results are presented as median and interquartile range (IQR) or number and percent unless otherwise noted. Demographic variables in infants with and without ASD (Table 1) were compared with Wilcoxon rank-sum test for continuous variables and Fisher’s exact test (two sided) for categorical variables.
Area under the receiver operator characteristics (ROC) curve was calculated for the four HR metrics in infants with and without eventual diagnosis of ASD. The significance of HR metrics for predicting ASD was further evaluated with a multivariate logistic regression model correcting for baseline variables and postmenstrual age (PMA; Table 2). Baseline variables included in the modeling were sex, gestational age (GA), birth weight, and Trisomy 21.
In the cohort of infants with at least 4 days of HR data available for analysis, rate and relative risk of ASD diagnosis were determined for low, medium, and high percentiles of HR skewness (HRskw) in male and female infants (Table 3). Statistical significance was adjusted for repeated measures using Huber–White method for robust covariance estimation.32,33 Statistical analyses were performed in GraphPad Prism (GraphPad Software, San Diego, CA) and MATLAB (MathWorks, Natick, MA) with two-tailed p < 0.05 considered statistically significant.
Results
Demographics and clinical features of infants with and without eventual ASD diagnosis
From 2009 to 2016, 4762 infants were admitted to the UVa NICU (56% male, 58% premature at <37 weeks GA, and 10% extremely premature at <28 weeks GA). In total, 2371 (50%) of these infants met inclusion criteria of having archived HR data available for analysis and having been seen in the UVa Health System beyond 3 years of age (56% male, 61% premature, and 11% extremely premature). Compared to included infants, those excluded were similar in terms of race and ethnicity (both groups 19% Black and 7% Hispanic), median 5-min Apgar (both groups 8), median maternal age (27 years included vs. 28 excluded), and C-section delivery (52 vs. 46%).
Of the 2371 infants included in the HR analysis, 88 were diagnosed with ASD (4% prevalence, of which 74% were male). Demographics of infants with and without ASD are shown in Table 1. Twins constituted 24% of the ASD cohort (with 3 of the 8 twin sets concordant for having ASD), and twins or triplets constituted 16% of controls (p = 0.056). ASD was statistically significantly associated with male sex and Trisomy 21 (both p < 0.001). A multivariate logistic regression model including birth weight, GA, sex, and Trisomy 21 predicted future diagnosis of ASD with area under the ROC curve of 0.637. In this multivariate model, birth weight and GA were not statistically significant. Trisomy 21 and male sex had odds ratios (95% confidence interval) of 8.05 (3.65, 17.74) and 2.21 (1.36, 3.59) for ASD, respectively.
HR mean, standard deviation, skewness, and kurtosis in ASD
In total, 689,016 infant-hours of data were analyzed. Median number of hours of data analyzed per infant was 182 (IQR 63, 376) with no significant difference in data coverage for ASD and control infants. Mean and kurtosis of HR for the entire NICU stay were not significantly different in infants with and without ASD but HR standard deviation and skewness were, with skewness having the strongest association (Table 2).
Skewness reflects asymmetry of a HR histogram. Figure 1 shows representative 10-min HR tracings with low HRskw reflecting decelerations (Fig. 1a) and high HRskw reflecting accelerations (Fig. 1c). Of note, the skewness metric is very sensitive to outlier values, such that short sharp decelerations or accelerations drive the value down or up, respectively, whereas a gradual increase or decrease in HR does not cause a large shift in the skewness value (Fig. 1b).
Three panels show 10 min of raw HR data (top) and a histogram count of all 300 HR values in the segment (bottom), with arrows in a and c indicating outlier values. In a, the baseline HR is 150–170 beats per minute (bpm) with a large deceleration to about 70 bpm, resulting in a low skewness value of −4.06. In b, the HR gradually increases and decreases from about 150 to 200 bpm, with superimposed small accelerations and decelerations, resulting in a near-zero skewness value of −0.01. In c, the baseline HR is about 110–120 bpm with three sharp accelerations, resulting in a high skewness value of +2.63.
HRskw association with PMA and with ASD diagnosis
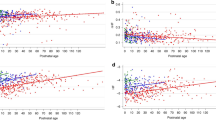
Figure 2 shows that HRskw is directly proportional to PMA and is associated with risk for ASD. Preterm infants have transient HR decelerations, in part due to apnea, reflected as negative skewness. We have previously published that apnea of prematurity diminishes significantly beyond 34 weeks PMA.34 In the current analysis, HRskw increased with advancing PMA, leveling off beyond 34 weeks PMA (Fig. 2a), and we therefore analyzed data beyond this maturational timepoint. A density plot of all hourly HRskw values from 34 to 42 weeks PMA (Fig. 2b) shows a shift toward higher values in the 88 infants with ASD compared to controls. There was a positive linear association between HRskw and relative risk of eventual diagnosis of ASD (Fig. 2c). Increased proportion of very high positive HRskw (>1) was observed in males with ASD diagnosis but not in females (Fig. 3).
For 2371 NICU patients, HR skewness was measured in 10-min segments and averaged hourly. a Mean HR skewness increases with advancing postmenstrual age from 26 to 34 weeks PMA, then levels off. At all PMAs, the 88 infants later diagnosed with ASD (dashed purple line) have higher mean HR skewness than infants without ASD (solid black line). The vertical dashed lines at 34 and 42 weeks PMA indicate the time frame represented in the other two panels. b From 34 to 42 weeks PMA, the relative density of hourly HR skewness measurements is shown for control infants (solid black line, 658,868 total hours) and for infants later diagnosed with ASD (dashed purple line, 30,148 total hours). The vertical dashed line indicates a very high HR skewness value of 1, which is further analyzed in Fig. 3. c With increasing mean hourly HR skewness, the relative risk (left y axis) and rate (right y axis) of ASD increases. The horizontal line at relative risk of 1 corresponds to the 4.4% rate of ASD weighted to adjust for variable amounts of data per infant between PMA 34 and 42 weeks.
Across a range of PMA from 26 to 42 weeks, percentage of HR skewness values >1 is shown for control infants (black line), males with ASD (solid blue circles), and females with ASD (open red triangles). Number of male infants with ASD represented at each week PMA from 34 to 42 ranged from 20 to 45 and number of females with ASD 6–15.
Since some infants were in the NICU for a very short time or had limited HR data available for analysis that might affect results, we performed analyses focused on infants with at least 4 days of HR data from 34 to 42 weeks PMA (n = 1618, 47 males and 17 females with ASD). For this subgroup, we calculated the average HRskw as a single measurement to assess risk of ASD. The univariate AUC (p values) for this metric are 0.570 (0.06), 0.600 (0.02), and 0.524 (0.73), respectively, for all, males, and females. Average HRskw is also significant (0.008) when correcting for baseline risk factors and has an AUC of 0.664 compared to 0.637 with only baseline risk factors. For male infants in the highest 5th percentile of HRskw measurements, rate of ASD diagnosis was 11.1%, whereas in the overall population of males in that analysis the rate was 5.1%, as shown in Table 3. In contrast, males in the lowest 25th percentile for HRskw measurements had about half the risk of ASD diagnosis. For females, the overall rate of ASD was 2.5% and the rate of those in the highest 5th percentile of HRskw was 3.7%.
Discussion
In this retrospective single-center cohort of NICU patients followed beyond 3 years of age, we found that high HRskw toward more short, sharp accelerations in the neonatal period was associated with increased risk for eventual diagnosis of ASD in males but not in females. This could reflect imbalance of the autonomic nervous system, which has been reported in children with ASD. If borne out in larger, multicenter cohorts, HR analysis might help to identify highest-risk infants for targeted screening and early therapies after NICU discharge.
This is the first report of a HR pattern during the NICU stay associated with future diagnosis of ASD. HR is regulated, in response to internal and external stimuli, through release of norepinephrine leading to accelerations and acetylcholine leading to decelerations through effects on pacemaker cells in the sinoatrial node of the heart. Maturation of autonomic responses occurs throughout gestation35,36 and in the neonatal period,37,38 with a shift from more sympathetic to more parasympathetic drive. Autonomic imbalance occurs in a variety of pathologic conditions and has been reported in children with ASD.16,18,20,21,22,23,25 Some studies have reported a slightly higher HR in children with ASD compared to typically developing children, especially during certain activities or phases of sleep.23 We did not find a difference in the mean HR in ASD compared to non-ASD infants throughout the NICU stay but were not able to account for sleep state. Another finding reported in children with ASD compared with neurotypical children is lower HR variability (HRV), generally characterized as decreased parasympathetic activity as indicated by the high-frequency component of HRV. High-frequency HRV measurement, reflecting respiratory sinus arrhythmia or acceleration during inspiration, is not possible to accurately measure in NICU infants who have high and irregular respiratory rates.39 We did not specifically measure HRV in our cohort, but we did find a higher standard deviation of HR in infants with autism.
Our major finding of increased HRskw being associated with increased risk of later diagnosis of ASD, and low HRskw with lower risk, is novel. As exemplified in Fig. 1, skewness can generally be thought of as asymmetry of a histogram of HR, with decelerations skewing the histogram to the left (negative values) and accelerations to the right (positive values). Notably, it is short, sharp decelerations or accelerations that significantly drive the HRskw measurement down or up, whereas more gradual accelerations and decelerations do not.
In our cohort, the fact that HRskw increased from negative toward zero with advancing PMA (Fig. 2A) should not come as a surprise to clinicians who care for preterm infants. Apnea of prematurity is often associated with HR decelerations, and apnea declines after about 34 weeks PMA.34 To account for this normal developmental maturation, we focused our analysis of ASD risk on the period from 34 to 42 weeks PMA. We found that male infants eventually diagnosed with ASD were overrepresented in the group of infants with very high HRskw (Fig. 3). Since this is a retrospective study, we are not able to account for the numerous clinical variables that can impact HR; however, we speculate that increased accelerations represent either sympathetic overactivity or parasympathetic (vagal) underactivity. Prospective studies are needed to address internal or external variables that might lead to intermittent tachycardia and also to characterize the height, duration, frequency, and timing of HR accelerations in order to begin to elucidate the mechanism of this finding. It is particularly intriguing that females with ASD in our cohort (albeit a small number) did not display high HRskw, further highlighting sex differences in pathophysiology of this condition. It is also interesting to note that a lower HRskw from 34 to 42 weeks PMA (beyond the period of apnea-related bradycardia) was associated with lower risk of ASD (Fig. 2c and Table 3). We can only speculate that this might reflect better parasympathetic nervous system function.
Strengths of this study include a large sample size and a similar prevalence of ASD as in other studies of high-risk populations of preterm and sick full-term infants (4%) and a similar sex distribution (3:1). Importantly, our non-ASD control group includes patients with other significant neurodevelopmental diagnoses, such as global developmental delay, attention deficit hyperactivity disorder, and cerebral palsy. This suggests that high HRskw is not a non-specific risk indicator of any neurodevelopmental disability. Although we included infants with Trisomy 21, who are known to have both autonomic instability and high risk of ASD,40 the HRskw finding persisted after adjusting for Trisomy 21.
Limitations of this study include the low number of females with ASD and our inability to exclude the possibility of more subtle ASD or diagnosis of ASD beyond the most recent age seen in our healthcare system, which was as young as 3 years. Although diagnosis of ASD was generally made by UVa developmental pediatrics specialists, cases were only ascertained through retrospective chart review and were not independently confirmed by standardized autism tests. A limitation with regard to the HR analysis is that we did not attempt to remove potential motion artifact from the electrocardiogram signal. Additionally, we are unable to determine whether high HRskw occurs during particular activities or in response to environmental stimuli. Research in children with autism suggests that abnormalities of autonomic activation reflected in HR patterns are particularly found in certain phases of sleep or in response to visual or auditory stimuli, which we were not able to assess in our retrospective study. These are all important considerations in future studies of HR patterns in neonates in relation to later ASD diagnosis.
Risk assessment during the NICU stay might be beneficial for identifying, among the inherently high-risk NICU population, those at especially high risk of eventual diagnosis of ASD. Currently, the median age of ASD diagnosis is about 4 years,1 but emerging research suggests that earlier therapeutic interventions during the period of developing neural connectivity could lead to improved outcomes.13,41,42 Narrowing this age gap is difficult without reliable biomarkers.12,43 The results we present are novel and require a great deal more investigation before determining whether and how they could be useful in clinical practice. Other than expanding this research to other NICUs, consideration should be given to increasing the number and types of HR algorithm testing, measuring HR responses to specific sensory stimuli, determining the optimum age and duration of monitoring for ASD prediction, and determining whether specific ASD phenotypes are more likely to be associated with atypical HR patterns in the neonatal period.
Conclusion
For male infants requiring admission to the NICU, a pattern of high HRskw reflecting more HR accelerations is associated with increased risk for later diagnosis of ASD. We speculate that this represents aberrant autonomic activation. Further research is warranted to determine whether HR analysis in the neonatal period can serve as a biomarker for autism risk and facilitate more comprehensive screening for autistic features very early in life when interventions could have the biggest impact.
References
Baio, J. et al. Prevalence of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2014. MMWR Surveill. Summ. 67, 1–23 (2018).
Agrawal, S., Rao, S. C., Bulsara, M. K. & Patole, S. K. Prevalence of autism spectrum disorder in preterm infants: a meta-analysis. Pediatrics 142, e20180134 (2018).
Meldrum, S. J. et al. Autism spectrum disorder in children born preterm-role of exposure to perinatal inflammation. Front. Neurosci. 7, 123 (2013).
van Tilborg, E. et al. Combined fetal inflammation and postnatal hypoxia causes myelin deficits and autism-like behavior in a rat model of diffuse white matter injury. Glia 66, 78–93 (2018).
Getahun, D. et al. Association of perinatal risk factors with autism spectrum disorder. Am. J. Perinatol. 34, 295–304 (2017).
Lindquist, B., Carlsson, G., Persson, E.-K. & Uvebrant, P. Behavioural problems and autism in children with hydrocephalus: a population-based study. Eur. Child Adolesc. Psychiatry 15, 214–219 (2006).
DiGuiseppi, C. et al. Screening for autism spectrum disorders in children with Down syndrome: population prevalence and screening test characteristics. J. Dev. Behav. Pediatr. 31, 181–191 (2010).
Sigmon, E. R., Kelleman, M., Susi, A., Nylund, C. M. & Oster, M. E. Congenital heart disease and autism: a case-control study. Pediatrics 144, e20184114 (2019).
Xiang, A. H. et al. Association of maternal diabetes with autism in offspring. JAMA 313, 1425–1434 (2015).
Winkler-Schwartz, A., Garfinkle, J. & Shevell, M. I. Autism spectrum disorder in a term birth neonatal intensive care unit population. Pediatr. Neurol. 51, 776–780 (2014).
Marino, B. S. et al. Neurodevelopmental outcomes in children with congenital heart disease: evaluation and management: a scientific statement from the American Heart Association. Circulation 126, 1143–1172 (2012).
Pelphrey, K. Charting a course for autism biomarkers. Biol. Psychiatry 82, 155–156 (2017).
MacDonald, R., Parry-Cruwys, D., Dupere, S. & Ahearn, W. Assessing progress and outcome of early intensive behavioral intervention for toddlers with autism. Res Dev. Disabil. 35, 3632–3644 (2014).
Rogers, S. J. & Vismara, L. A. Evidence-based comprehensive treatments for early autism. J. Clin. Child Adolesc. Psychol. 37, 8–38 (2008).
Axelrod, F. B., Chelimsky, G. G. & Weese-Mayer, D. E. Pediatric autonomic disorders. Pediatrics 118, 309–321 (2006).
Bujnakova, I. et al. Autism spectrum disorder is associated with autonomic underarousal. Physiol. Res. 65, S673–S682 (2016).
Daluwatte, C. et al. Atypical pupillary light reflex and heart rate variability in children with autism spectrum disorder. J. Autism Dev. Disord. 43, 1910–1925 (2013).
Bharath, R., Moodithaya, S. S., Bhat, S. U., Mirajkar, A. M. & Shetty, S. B. Comparison of physiological and biochemical autonomic indices in children with and without autism spectrum disorders. Medicina 55, 346 (2019).
Billeci, L. et al. Heart rate variability during a joint attention task in toddlers with autism spectrum disorders. Front. Physiol. 9, 467 (2018).
Cheshire, W. P. Highlights in clinical autonomic neuroscience: new insights into autonomic dysfunction in autism. Auton. Neurosci. 171, 4–7 (2012).
Sheinkopf, S. J. et al. Developmental trajectories of autonomic functioning in autism from birth to early childhood. Biol. Psychol. 142, 13–18 (2019).
Ming, X., Patel, R., Kang, V., Chokroverty, S. & Julu, P. O. Respiratory and autonomic dysfunction in children with autism spectrum disorders. Brain Dev. 38, 225–232 (2016).
Harder, R. et al. Heart rate variability during sleep in children with autism spectrum disorder. Clin. Auton. Res 26, 423–432 (2016).
Schaaf, R. C., Benevides, T. W., Leiby, B. E. & Sendecki, J. A. Autonomic dysregulation during sensory stimulation in children with autism spectrum disorder. J. Autism Dev. Disord. 45, 461–472 (2015).
Benevides, T. W. & Lane, S. J. A review of cardiac autonomic measures: considerations for examination of physiological response in children with autism spectrum disorder. J. Autism Dev. Disord. 45, 560–575 (2015).
Perdue, K. L., Edwards, L. A., Tager-Flusberg, H. & Nelson, C. A. Differing developmental trajectories in heart rate responses to speech stimuli in infants at high and low risk for autism spectrum disorder. J. Autism Dev. Disord. 47, 2434–2442 (2017).
Fairchild, K. D. et al. Vital signs and their cross-correlation in sepsis and NEC: a study of 1,065 very-low-birth-weight infants in two NICUs. Pediatr. Res. 81, 315–321 (2017).
Sullivan, B. A. et al. Early pulse oximetry data improves prediction of death and adverse outcomes in a two-center cohort of very low birth weight infants. Am. J. Perinatol. 35, 1331–1338 (2018).
Vesoulis, Z. A. et al. Early hypoxemia burden is strongly associated with severe intracranial hemorrhage in preterm infants. J. Perinatol. 39, 48–53 (2019).
Fairchild, K. D. et al. Abnormal heart rate characteristics are associated with abnormal neuroimaging and outcomes in extremely low birth weight infants. J. Perinatol. 34, 375–379 (2014).
Vergales, B. D. et al. Depressed heart rate variability is associated with abnormal EEG, MRI, and death in neonates with hypoxic ischemic encephalopathy. Am. J. Perinatol. 31, 855–862 (2014).
Harrell, F. E. in Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis 181–217 (Springer, 2015).
White, H. Maximum likelihood estimation of misspecified models. Econometrica 50, 1 (1982).
Fairchild, K. et al. Clinical associations of immature breathing in preterm infants: part 1-central apnea. Pediatr. Res. 80, 21–27 (2016).
Schneider, U. et al. Developmental milestones of the autonomic nervous system revealed via longitudinal monitoring of fetal heart rate variability. PLoS ONE 13, e0200799 (2018).
Burtchen, N. et al. Autonomic signatures of late preterm, early term, and full term neonates during early postnatal life. Early Hum. Dev. 137, 104817 (2019).
Sahni, R. et al. Maturational changes in heart rate and heart rate variability in low birth weight infants. Dev. Psychobiol. 37, 73–81 (2000).
Porges, S. W. & Furman, S. A. The early development of the autonomic nervous system provides a neural platform for social behaviour: a polyvagal perspective. Infant Child Dev. 20, 106–118 (2011).
Rother, M., Witte, H., Zwiener, U., Eiselt, M. & Fischer, P. Cardiac aliasing—a possible cause for the misinterpretation of cardiorespirographic data in neonates. Early Hum. Dev. 20, 1–12 (1989).
Carvalho, T. Dde et al. Heart rate variability in individuals with Down syndrome - a systematic review and meta-analysis. Auton. Neurosci. 213, 23–33 (2018).
Dawson, G. & Bernier, R. A quarter century of progress on the early detection and treatment of autism spectrum disorder. Dev. Psychopathol. 25, 1455–1472 (2013).
Douglas, P. S. Pre-emptive intervention for autism spectrum disorder: theoretical foundations and clinical translation. Front. Integr. Neurosci. 13, 66 (2019).
McPartland, J. C. et al. The Autism Biomarkers Consortium for Clinical Trials (ABC-CT): scientific context, study design, and progress toward biomarker qualification. Front. Integr. Neurosci. 14, 16 (2020).
Acknowledgements
We would like to thank Kevin Pelphrey for helpful commentary in preparation of the manuscript. This study was funded by the NIH grant HD072071.
Author information
Authors and Affiliations
Contributions
All authors have met the Pediatric Research authorship requirements. All authors had substantial contribution to conception and design, acquisition of data, or analysis and interpretation of data. All authors helped draft the article, revise it critically for important intellectual content, and have approved this final version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent
Patient consent was not required for this retrospective study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Blackard, K.R., Krahn, K.N., Andris, R.T. et al. Autism risk in neonatal intensive care unit patients associated with novel heart rate patterns. Pediatr Res 90, 1186–1192 (2021). https://doi.org/10.1038/s41390-021-01381-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-021-01381-1
This article is cited by
-
Neonatal autonomic regulation as a predictor of autism symptoms in very preterm infants
Journal of Perinatology (2024)
-
Heart rate patterns predicting cerebral palsy in preterm infants
Pediatric Research (2023)