Abstract
Background
Infants with advanced necrotizing enterocolitis (NEC) often need surgical resection of necrotic bowel. We hypothesized that incomplete resection of NEC lesions, signified by the detection of necrotic patches in margins of resected bowel loops, results in inferior clinical outcomes.
Methods
We reviewed the medical records of infants with surgical NEC in the past 15 years for demographic, clinical, and histopathological data. We also developed statistical models to predict mortality and hospital stay.
Results
Ninety infants with surgical NEC had a mean (±standard error) gestational age of 27.3 ± 0.4 weeks, birth weight 1008 ± 48 g, NEC onset at 25.2 ± 2.4 days, and resected bowel length of 29.2 ± 3.2 cm. Seventeen (18.9%) infants who had complete resection of the necrosed bowel had fewer (4; 23.5%) deaths and shorter lengths of hospital stay. In contrast, a group of 73 infants with some necrosis within the margins of resected bowel had significantly more (34; 46.6%) deaths and longer hospital stay. The combination of clinical and histopathological data gave better regression models for mortality and hospital stay.
Conclusion
In surgical NEC, incomplete resection of necrotic bowel increased mortality and the duration of hospitalization. Regression models combining clinical and histopathological data were more accurate for mortality and the length of hospital stay.
Impact
-
In infants with surgical NEC, complete resection of necrotic bowel reduced mortality and hospital stay.
-
Regression models combining clinical and histopathological information were superior at predicting mortality and hospital stay than simpler models focusing on either of these two sets of data alone.
-
Prediction of mortality improved with the combination of antenatal steroids, chorioamnionitis, and duration of post-operative ileus, with severity of inflammation and hemorrhages in resected intestine.
-
Length of hospital stay was shorter in infants with higher gestational ages, but longer in those with greater depth of necrosis or needing prolonged parenteral nutrition or supervised feedings.
Similar content being viewed by others
Introduction
Necrotizing enterocolitis (NEC) is an inflammatory bowel necrosis seen in 5–10% of very-low-birth-weight infants born between 22 and 28 weeks gestation.1 The disease remains a leading cause of morbidity and mortality, surgical intervention, and prolonged medical/nursing needs in the surviving infants. The etiopathogenesis is unknown; the occurrence of NEC may be higher in infants with enteric dysbiosis and overgrowth of Gram-negative bacteria.2,3,4 The risk of developing NEC may increase following hypoxia and/or hypothermia, with limited anti-microbial defenses due to immature Paneth cells, due to severe anemia and/or red blood cell transfusions, or following enteral exposure to immunological stimuli.4,5,6,7,8
Infants with advanced NEC need intensive care, and if stable enough to withstand general anesthesia and surgery, may undergo an exploratory laparotomy.9 The necrotic segments of bowel are resected and the remaining intestine may be managed with primary anastomoses and/or intestinal stomata.5,10,11,12 Histopathologically, the resected bowel typically shows coagulation necrosis, inflammation, interstitial hemorrhages, and reparative changes.13,14,15,16,17,18,19 These changes typically begin at the mucosa and progress outward towards the serosa. The severity of these pathological abnormalities may correlate with the disease severity, the pace of progression, and may even help predict the outcomes.20,21
In this study, we hypothesized that infants with surgical NEC were predisposed to adverse post-operative outcomes if they had incomplete removal of the diseased bowel, as indicated by the detection of necrotic changes in the margins of their resected intestine. This incomplete removal of the necrotic bowel could possibly delay recovery due to bacterial translocation and inflammation. Using a clinical–pathological approach, we first examined the medical records of the enlisted infants for their demographic information and clinical course. Surgical specimens were then examined to (a) confirm the diagnosis and grade the severity of necrosis; and (b) check resection margins to ascertain that all NEC-affected bowel had been completely removed.
Methods
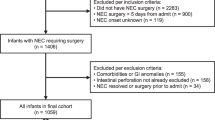
This retrospective study was conducted at the University of Mississippi Medical Center (UMMC) at Jackson, Mississippi, after approval for patient rights by the Institutional Review Board and for compliance with the Health Insurance Portability and Accountability Act of 1996. The study was conducted at the UMMC neonatal intensive care unit (NICU), which is a regional referral center for infants with surgical NEC. A detailed review of the medical records identified 118 patients who underwent exploratory laparotomy for advanced NEC during the period between January 2000 and December 2015.9 After excluding nine infants who were missing important clinical data, and 19 with confounding disorders, such as congenital heart disease, intestinal atresia, and spontaneous intestinal perforation (SIP), we identified 90 infants as eligible for this study (Fig. 1).
Clinical information
Demographic data such as birth weight, gestational age, gender, ethnicity (African-American, Caucasian, or Latino), and mode of delivery were noted. Other important clinical information included Apgar scores ≤6 at 5 min, age at initiation of feedings, culture-proven sepsis prior to NEC, central line use, patent ductus arteriosus, indomethacin therapy, and the age of onset and the clinical presentation of NEC (abdominal distension, bloody stools, and/or pre-feeding residuals ≥30% of the feeding volume).
Our primary clinical outcomes were the length of hospital stay and mortality from NEC. The surgical interventions described the length and specific region(s) of the intestine. The post-operative morbidities included the duration of paralytic ileus, pressor support for ≥24 h after surgery, and days of antibiotic treatment. The duration of parenteral nutrition was noted as the number of hospital days from surgery until they achieved 120 mL/kg/day of enteral feeding. Surgical complications and stoma creation were recorded. Short bowel syndrome was defined as the need for parenteral nutrition at discharge.
Pathology
Hematoxylin and eosin (H&E)-stained sections of surgically resected intestine were evaluated by two blinded pathologists, one each from the University of Mississippi and the University of Alabama, Birmingham. We evaluated the resected bowel for (a) histopathological evidence of NEC, including coagulative necrosis, inflammation, hemorrhages, and reparative changes; and (b) completeness of the surgical resection of the intestine affected by NEC, evident by the healthy appearance and the absence of any necrotic/inflamed foci in the edges of the resected bowel. Two sections from both the proximal and distal edges were evaluated. Coagulative necrosis in intestinal cells was defined by the loss of hematoxylin staining of the nuclei and diminished cytoplasmic staining with eosin, but with relatively preserved, “ghost-like” crypt–villus histoarchitecture. Inflammation was noted as infiltration by inflammatory cells, such as monocytes and neutrophils. White blood cell (WBC) counts were enumerated per high-power field (HPF) in the four layers of the intestine in NEC lesions. At least 10 HPFs were evaluated per specimen. Hemorrhages, marked by the extravasation of blood cells, were recorded in various layers of the intestine. Reparative changes included microscopic evidence of neo-vascularization, increased fibroblasts or myofibroblasts, and epithelial regeneration. The severity of NEC was assessed by the depth to which these histopathological changes (coagulative necrosis, inflammation, hemorrhages, and reparative changes) were seen: grade 1 was limited to the mucosa, grade 2 changes extended to the submucosa, grade 3 to the muscularis, and grade 4 change was transmural. The resection margins were evaluated by the depth up to which these changes were seen: group 1 was defined by 0–5% involvement of the margins, group 2 of 5–20%, group 3 of 20–50%, and a group 4 of >50%. Figure 2 shows representative histopathological findings in surgically resected bowel loops.
These images show a <5% necrosis, 20% inflammation, and 30% hemorrhage; b fibrinous exudate, 15% necrosis, 20% inflammation, and congested vessels; c 70% necrosis, 40% inflammation, and 10% hemorrhage, and some vascular congestion. d shows ileum resected from an infant with NEC. The rectangle is placed to highlight villus epithelial cells with regenerative changes. These cells show nuclear stratification and hyperchromasia, and amphophilic staining of the cytoplasm with both acid and basic dyes. Magnification bars = 150 µm.
Statistical information
Statistical analyses were performed using the software programs STATA 15 (Stata Statistics, College Station, TX) and/or GraphPad Prism (GraphPad Software, San Diego, CA). Descriptive statistics were computed; categorical data were summarized as absolute and relative frequencies and tested for differences using χ2 tests. Continuous data were presented as means with standard errors (SE) when distributions were symmetric or as medians (with first and third quartiles) when a skew was noted. Differences in continuous data were tested using a Student’s t test, or if the symmetry was in question, by a Mann–Whitney U test. Mortality, length of hospital stay, and other variables were analyzed using a generalized linear multivariable gamma model with and without adjustment for birth weight, gestation, gender, and mortality. Parameter estimates were computed by the least-squares methods, with estimates, standard errors, and the 95% confidence intervals (CIs). T values were used to compute the size of the difference relative to the variation in the sample data. Data were evaluated for normality of residuals using the Anderson–Darling, D’Agostino–Pearson, Shapiro–Wilk, and Kolmogorov–Smirnov tests. Differences were considered significant at p < 0.05.
Results
Clinical information
We enrolled 90 patients in this study (Fig. 1), most of whom have been described previously.9 We correlated their clinical information and the histopathological characteristics of their resected bowel. These infants were born at (mean ± SEM) of 27.3 ± 0.4 weeks with a birth weight of 1008 ± 48 g. Forty (44.4%) infants were outborn. Fifty-six (62.2%) were male. Eighty (88.9%) were African American, 9 (10%) were Caucasian, and 1 (1.1%) was Latino. NEC occurred on 25.2 ± 2.4 days; 83 (92%) presented with abdominal distension, 4 (4.5%) with feeding intolerance, and 3 (3.5%) with bloody stools.
All enrolled infants had radiological indicators of NEC such as pneumatosis, dilated bowel, and/or other signs of ileus. These patients underwent an exploratory laparotomy upon the onset of increasing abdominal distention, persistent hypotension and/or metabolic acidosis, or the need for repeated red blood cell transfusions. In addition to the radiological and clinical signs, NEC was further confirmed with intra-operative and pathological examination of the bowel. Histopathological findings of NEC were seen in the resected small intestine (ileum or jejunum) in 65 (72%), small and large intestine in 24 (27%), and the colon in one infant. Post-operative ileus lasted 16.5 ± 1.3 days, and 55 (61.1%) infants needed extra intravenous fluids and pressor support. Thirty-eight (42.2%) patients died at 102.6 ± 14 days, which was shorter than the discharge age of 153.5 ± 11 days in the 52 (57.8%) survivors. Table 1 summarizes the clinical characteristics of the included infants and their mothers.
Pathology
We reviewed 250 intestinal specimens resected from our 90 patients. Each specimen was first examined with serial sections to estimate the histopathological severity of NEC, focusing on necrosis, inflammation, hemorrhages, and reparative changes. Then, we examined the edges to either confirm a clean resection, or alternatively, estimate the depth to which the resection margins showed signs of NEC.
Necrosis
Intestinal loops resected from 17 infants (18.89%) had clean resection margins with no necrosis. These infants were classified into group A. The maximum necrosis grading in bowel loops resected from these infants was 2.31 ± 1.38. Group B included 29 infants (32.22%) whose resected bowel loops showed necrosis to a depth of 9.14 ± 0.61% in the margins, and a maximum necrosis grading of 2.03 ± 0.25. Group C with 27 (30%) infants had 26.85 ± 1.58% necrosis in the margins and a maximum necrosis grading of 1.41 ± 0.21, and group D had 17 infants (18.9%) with 67.65 ± 3.59% necrosis in the resection margins and a necrosis grading of 1.53 ± 0.26. Compared to group A, groups B–D showed significantly increased necrosis in the resection margins (p < 0.001). There was a trend for reduction in the maximum severity of necrosis in the resected bowel loop, but these differences did not reach significance (p = 0.08).
The length of the resected intestine in group A was 14.98 ± 2.42 cm. Intestinal resection in group B was 30 ± 6.65 cm, which seemed longer but did not reach significance (p = 0.09). The length of the resected bowel was 33.65 ± 6.65 cm (p = 0.018) in group C and 34.79 ± 8.08 cm (p = 0.025) in group D. The depth of necrosis in the resection edges correlated negatively with reparative changes (r = −0.28, p = 0.008). The severity of necrosis in the intestinal segment correlated with inflammation (r = 0.38, p < 0.001) and hemorrhages (r = 0.37, p < 0.001).
Inflammation
The severity of inflammation in groups A–D was of grades 2.71 ± 0.28, 2.38 ± 0.23, 2.7 ± 0.1, and 3.06 ± 0.2, respectively. Inflammation was seen in 14.12 ± 2.36%, 23.97 ± 2.25%, 23.7 ± 2.35%, and 23.53 ± 2.09% of the resected margins (p = 0.025 in groups A vs. B, and p = 0.036 in groups A vs. C). There were 52 ± 9.5, 87.3 ± 8.8, 98 ± 8.9, and 86.3 ± 13 WBCs/HPF, respectively, in the four groups (p = 0.01). Besides the aforementioned association with necrosis, inflammation also correlated with hemorrhages (r = 0.52, p < 0.001) and gestational age (r = 0.21, p = 0.05). There were negative relationships with antenatal steroids (r = −0.27, p = 0.012) and days on PN (r = −0.27, p = 0.035), and negative trends with the length of hospitalization (r = −0.2, p = 0.064) and reparative changes in the resected intestine (r = −0.18, p = 0.086).
Hemorrhages
Hemorrhages were seen in 89 infants. Two infants had small bleeds, and larger hemorrhages were seen in 87. The severity of hemorrhages are shown in Table 2. In the four groups based on the severity of necrosis in resection margins, hemorrhages were seen in 12.35 ± 2.27%, 20.34 ± 2.12%, 23.33 ± 1.19%, and 17.94 ± 2.94% (group A vs. B, p = 0.02; group A vs. C, p = 0.03). In addition to the association with inflammation, there were negative trends with antenatal steroids (r = −0.18, p = 0.08) and reparative changes (r = −0.18, p = 0.091).
Reparative changes
Twenty-four of our 90 patients (26.67%) showed reparative changes. These changes correlated with hemorrhages (r = 0.21, p = 0.046) and with indomethacin use (r = 0.22, p = 0.048). There was also a trend for correlation with mortality (r = 0.19, p = 0.076).
In the resection margins, the average grades of reparative changes were 0.24 ± 0.16, 0.62 ± 0.21, 0.27 ± 0.1, and 1.24 ± 0.3 (group A vs. D, p = 0.02; group C vs. D, p = 0.01). The overall grades in the four groups seemed artificially lower because reparative changes were seen only in a minority of infants. In margins, group B showed overall stronger reparative changes than group D (3.08 ± 1.75% vs. 0 ± 0%; p = 0.02), and a trend for stronger changes than group C (p = 0.056). Group C seemed to have stronger changes in the resection margins than group D (p = 0.06).
Clinical–pathological correlation
The clinical and pathological data are described below and in Table 2.
Mortality
Infants who died of NEC (n = 38, 42.2%) were born at 26.11 ± 0.48 weeks and weighed 862.76 ± 52.48 g, compared to the survivors who were born slightly later at 28.1 ± 0.5 weeks (p = 0.033) and weighed 1120.39 ± 70.41 g (p = 0.008). Gender distribution (16/38 female fatalities vs. 18/52 female survivors), SGA (6/38 deaths vs. 8/52 survivors), and the onset of NEC (27.7 ± 4.3 vs. 23.3 ± 2.7 days) did not affect mortality. Infants who died of NEC lost more bowel (37.5 ± 6.04 vs. 23.4 ± 3.19 cm; p = 0.03) and had shorter hospital stays (101 ± 14 vs. 156 ± 14 days; p = 0.002). They showed a trend to reach full feedings earlier (54.42 ± 5.64 vs. 81.86 ± 6.17 days, p = 0.06) with fewer days of feedings (39.82 ± 5.2 vs. 64.81 days, p = 0.08). The demographic and clinical characteristics of infants who survived vs. those who died are summarized in Table 3.
Linear regression for NEC-related mortality (r2 = 0.56, p = 0.03) identified several pre-surgical factors such as PDA (|t| = 3.09; p = 0.005), chorioamnionitis (|t| = 2.77; p = 0.011), and PIH (|t| = 2.45; p = 0.023). After surgery, the duration of paralytic ileus (|t| = 3.03; p = 0.006), days of starting (|t| = 3.02; p = 0.006) and reaching full feedings (|t| = 2.7; p = 0.013), and days of antibiotics (|t| = 2.49; p = 0.02) were important contributors. The prediction of mortality improved considerably (r2 = 0.90, p < 0.001) after including pathological data, such as inflammation (|t| = 3.33; p = 0.004) and hemorrhages (|t| = 6.24; p < 0.001) in the resected intestine. In this combined model, post-operative ileus (|t| = 6.82, p < 0.001), day of starting enteral feedings (|t| = 6.82, p = 0.014), antenatal steroids (|t| = 5.74, p < 0.001), PIH (|t| = 5.04; p < 0.001), chorioamnionitis (|t| = 2.72, p = 0.04), outborn status (|t| = 3.88, p = 0.001), and the time of achievement of full feedings (|t| = 2.24, p = 0.039) remained important.
Length of stay
The average length of stay (LOS) was 132.67 ± 9.09 days. Infants with higher gestational ages (r = −0.28, p = 0.008) and birth weight (r = −0.29, p = 0.006) needed fewer intensive care days. However, longer stays were needed for infants with higher chronological age at onset of NEC (r = 0.35, p = 0.001), those who needed PN for longer (r = 0.38, p < 0.001), or if they took longer to reach full feedings (r = 0.53, p < 0.001). Death was an important negative determinant (100.95 ± 13.94 days in those who died vs. 155.85 ± 10.92 days in survivors, p = 0.002). Table 4 highlights the correlation between the LOS with major outcomes.
Linear regression for LOS (r2 = 0.44; p < 0.001) identified the contribution of the age of onset of NEC (|t| =4.75; p < 0.001), mortality (|t| = 4.14; p < 0.001), gestational age (|t| =3.95; p < 0.001), days of PN (|t| = 3.72; p < 0.001), and birth weight (|t| = 2.79; p = 0.007). Pathological factors improved the model (r2 = 0.5, p < 0.001); the new factors were the severity grade of NEC in the resected segment (|t| = 2.2; p = 0.03) and the depth of necrosis (percent) at the resection edges (|t| = 1.98; p = 0.05). Trends were seen for associations with inflammation (p = 0.064).
Severity of necrosis
Bowel specimens from 17 infants showed clean, non-necrotic margins, compared to 73 patients with ≥grade 2 lesions and 29.3 ± 3% necrosis at the margins (p < 0.001). The resection margins in the former group were less inflamed (12.4 ± 2.3% vs. 20.9 ± 1.4%; p = 0.003) and had fewer hemorrhages (14 ± 2.4% vs. 23.8 ± 1.3%, p < 0.001). Linear regression (r2 = 0.1, p = 0.03) identified clinical determinants, such as gestational age (|t| =4.98, p < 0.001), gender (|t| = 2.27, p = 0.026), and indomethacin use (|t| = 2.31, p = 0.023). The accuracy of these models increased upon the inclusion of pathology findings (r2 = 0.79, p < 0.001), such as necrosis (%) in the resection margins (|t| = 17.32, p < 0.001). The clinical determinants were gestational age (|t| = 4.98, p < 0.001) and gender (|t| = 3.38, p = 0.001).
Length of resected bowel
The length of the resected bowel increased from group A through group D. Linear regression identified several clinical determinants of this length of bowel resection, but the accuracy of these models was limited (r2 = 0.16, p = 0.003). The clinical determinants in these models included birth weight (|t| = 3.56, p < 0.001), PDA (|t| = 2.76, p = 0.007), and assisted ventilation (|t| = 2.47, p = 0.016). The accuracy of these models improved (r2 = 0.35, p < 0.001) when the depth of necrosis in the resection margins (|t| = 4.22, p < 0.001) was included. These combined models included the age at onset of NEC (|t| = 2.52, p = 0.014), PDA (|t| = 3.27, p = 0.002), region of resected bowel (|t| = 4.39, p = 0.027), and assisted ventilation (|t| = 2.26, p = 0.027).
Discussion
We studied a cohort of 90 infants with surgical NEC to examine the hypothesis that incomplete surgical removal of the necrosed bowel might predispose them to adverse outcomes due to continued systemic inflammation. We recorded their perinatal data, intra- and post-operative events, clinical course, and outcomes, such as mortality and length of hospital stay, and correlated these data with the histopathological changes in the intestine. These infants had a clinical profile typical of NEC patients with a gestational age of ~27 weeks and a birth weight of 1000 g. The onset of NEC near postnatal day 25 was clinically typical with abdominal distension, feeding intolerance, and/or bloody stools. Post-operative ileus lasted for 16.5 ± 1.3 days, parenteral nutrition was given for 91 ± 7 days, feedings were started on 17 ± 2 days after surgery, and full feedings were achieved on 82 ± 6 days. Thirty-eight (42.2%) patients died. The average hospital stay was 132.5 ± 9 days.
We aimed to combine the clinical information of NEC patients with histopathological data from their surgically resected intestine, seeking to extend earlier attempts to connect the histopathology and outcomes of NEC.20,21 The pathology specimens were first evaluated for the overall severity of NEC. The resection margins were then specifically examined to either confirm a clean resection or, alternatively, evaluated for signs of NEC, such as necrosis, inflammation, hemorrhage, or reparative changes. In 17 infants, the necrotic bowel was resected cleanly with no NEC lesions seen in the resection margins. The other 73 infants showed notable necrosis and inflammation in the surgical margins. These infants had a considerably longer part of their bowel involved in NEC than those who had complete resection with clean margins. The presence of superficial NEC lesions near the resection margins most likely reflects the surgeons’ conscious efforts to salvage intestine that was not completely gangrenous. In premature infants, there may also have been some reluctance against the excision of bowel segments where necrosis/gangrene was not the certain diagnosis. Naked eye examination of the intestine may not be sufficient to identify segments with partial/focal hypoperfusion, thromboembolic foci, and transient ischemic–reperfusion injury even though such conditions could be considered in critically ill, older patients. Finally, necrotic and inflammatory lesions may evolve differently in extremely premature intestine than in full-term infants. Both premature leukocytes and the intestine have known functional limitations, which may add a maturational component to the temporal evolution of NEC lesions and may need further study.
In the resected intestine, leukocyte infiltration and inflammatory changes seemed to become more prominent with increasing severity of necrosis, and this relationship remained consistent in the resection margins. As in earlier studies, inflammation correlated with hemorrhages.10 Antenatal steroids and chronological age may also have protected against inflammation.11 Interstitial hemorrhages followed a similar pattern, and were possibly related to maturational changes in the microvasculature.12 These hemorrhages seemed to have been associated with necrosis and inflammation, which can be explained by the microvascular damage that has been documented in NEC.12,22 Another interesting variable was the reparative changes in the necrotic bowel segments.20,21 These changes may have been more prominent in mild-moderate NEC than in severe disease. There is a need to understand the regulatory mechanisms involved in these reparative changes.
Infants who died of NEC were born at an earlier gestational age (26.11 ± 0.48 vs. 28.1 ± 0.5 weeks) and with less birth weight (862.76 ± 52.48 vs. 1120.39 ± 70.41 g) than the survivors. The loss of bowel in these infants during surgery was nearly a third more than those who survived (37.5 ± 6.04 vs. 23.4 ± 3.19 cm). We also noted associations between death and longer paralytic ileus, difficulties with feeding, and infections. The prediction of mortality became highly accurate (r2 = 0.90, p < 0.001) with the addition of pathological data such as inflammation (p = 0.004) and hemorrhages (p < 0.001) in the resected intestine. Our data are consistent with previous reports that higher severity of necrosis may result in mortality.23,24 The non-survivors may have higher inflammatory scores. In an earlier small study,21 we had noted a similar association between transmural necrosis in the resected intestinal margins and increased mortality. Out of 22 infants (N = 33) studied, 6/8 (36.4%) had transmural necrosis and six of these eight (75%) infants died. In contrast, only 4/14 (28.6%) of the infants who did not have transmural necrosis in surgical margins died (p = 0.048). A recent report by Eaton et al.13 noted a modest inverse relationship between the histological severity of bowel necrosis and survival. They reported that infants with fatal outcomes expressed high levels of the markers of apoptosis, but not inflammation.
Our findings are similar to several past studies.14,15,16,17,18,19,25 Li et al.16 noted that infants with earlier onset of NEC had achieved full feedings earlier (means ± standard deviation 18.1 ± 11.5 vs. 26.3 ± 15.6 days, p = 0.008), had less mortality (p = 0.026), and showed a trend for fewer infections [19/53 (35.8%) vs. 29/53 (54.7%), CI: 0.212–1.008]. Sheng et al.14 reported that the timing of NEC in preterm infants was inversely related to the gestational age, need for surgical procedures, and survival. In another study, Wright et al.15 evaluated surgical NEC in 221 preterm neonates, where 182 (82%) underwent surgery in the operating room, but 15 (8%) were too sick to be transported and were operated at the bedside. They noted high mortality rates, particularly in those who could not be transported to the operating room.
Our cohort showed a higher frequency of NEC in African-American infants. Racial disparities in the incidence of NEC are known, but these ethnic differences need to be studied more comprehensively. The Pediatrix medical group recently reviewed the medical records of 126,089 infants (45% Caucasians, 27% African Americans, and 19% Latinos); NEC was noted in 8796 (7%).26 Compared to Caucasian infants, NEC was significantly more frequent in African Americans (adjusted odds ratios (AOR) 1.31, 95% CI [1.24–1.39] and Latinos (1.30 [1.21–1.39]). These African-American and Latino infants also had higher mortality (AORs 1.35 [1.15–1.58] and 1.31 [1.09-1.56], respectively) than Caucasians. Further studies are needed to investigate these health disparities and possible interventions to improve these health outcomes.
The average LOS in our study was 132.67 ± 9.09 days, and was shorter in more mature infants with higher gestational age and birth weight. In linear regression, gestational age, birth weight, age of onset of NEC, days of PN, and mortality were noted as important. The inclusion of pathological factors added to accuracy of the model by including the severity of NEC in the resected segment and the depth of necrosis (percent) at the resection edges. We noted better survival in NEC patients who had an average gestational age that was 3 weeks higher and birth weight that was higher by 250 g. These associations between maturity, illness, and survival are important.27 Both groups started enteral feedings at 16–17 days, but the survivors took a month longer to reach full feeding volumes and had longer hospitalizations. There was no difference in gender, ethnicity, clinical NEC, surgical complications, and short bowel syndrome. The effect of these demographic features and clinical practices on intestinal microflora remains unclear,21 but considering the increasing importance of microflora development in the causation of NEC,28,29,30 further study is needed to correlate these factors.
In our cohort, the length of resected bowel was a major indicator of clinical outcomes. The survivors lost less bowel than those who died (24 vs. 36 cm, p < 0.01). The ileocecal region was affected in a majority, which is consistent with previous reports.18,21 This region, particularly the ileum, is highly susceptible due to its low vascularity and high metabolic demands.31 Piglet studies show that the ileocecal region may be perfused at 40% lower levels than more proximal regions, and may be further compromised in lower birth weight animals with limited compensatory mechanisms.32,33,34 Studies conducted by the Eunice Kennedy Shriver National Institute of Child Health and Human Development have shown important associations between surgical NEC and SBS, with increased risk of neurodevelopmental abnormalities, repeated hospitalizations, and death.19
Our investigations suggest that incomplete excision of the necrotic bowel may lead to inferior outcomes in surgical NEC. In current care-management models, the involved surgeons, physicians, and service managers seek excision of the necrotic bowel in a procedure that is timely, feasible, and most likely to be tolerated by the critically ill infant. If findings in this report were to be confirmed, future studies may have to focus on the feasibility and costs of including pediatric pathology services in a procedure that is complex and usually emergent, but relatively infrequent at most centers. The easiest, most feasible solution may be to standardize the evaluation of frozen tissue sections for NEC in these infants. However, some exciting and potentially usable alternatives may also emerge from newer, semi-automated measurements of necrosis based on mass spectrometry, such as desorption electrospray ionization and imaging mass spectrometry.35,36
To conclude, we present novel clinical and pathological determinants of the outcomes in surgical NEC in this study. The limitations of this effort are its retrospective design, limited sample size, and single-center design, which increase the risk of bias. There is a need to validate these results in a larger, prospectively enrolled multi-centric cohort, which may also permit the inclusion of additional clinical/laboratory predictors in the statistical models.37,38,39,40 There is a need for continued evaluation of prognostic markers in infants with surgical NEC.
References
Neu, J. & Walker, W. A. Necrotizing enterocolitis. N. Engl. J. Med. 364, 255–264 (2011).
Ho, T. B. T. et al. Dichotomous development of the gut microbiome in preterm infants. Microbiome 6, 157 (2018).
Ho, T. B. T. et al. Enteric dysbiosis and fecal calprotectin expression in premature infants. Pediatr. Res. 85, 361–368 (2019).
Fundora, J. B., Guha P., Shores D. R., Pammi M. & Maheshwari A. Intestinal dysbiosis and necrotizing enterocolitis: assessment for causality using Bradford Hill criteria. Pediatr. Res. 87, 235–248 (2020).
MohanKumar, K. et al. Trinitrobenzene sulfonic acid-induced intestinal injury in neonatal mice activates transcriptional networks similar to those seen in human necrotizing enterocolitis. Pediatr. Res. 81, 99–112 (2016).
MohanKumar, K. et al. Gut mucosal injury in neonates is marked by macrophage infiltration in contrast to pleomorphic infiltrates in adult: evidence from an animal model. Am. J. Physiol. Gastrointest. Liver Physiol. 303, G93–G102 (2012).
Zhang, C. et al. Paneth cell ablation in the presence of Klebsiella pneumoniae induces necrotizing enterocolitis (NEC)-like injury in the small intestine of immature mice. Dis. Model. Mech. 5, 522–532 (2012).
MohanKumar, K. et al. A murine neonatal model of necrotizing enterocolitis caused by anemia and red blood cell transfusions. Nat. Commun. 10, 3494 (2019).
Garg, P. M., Hitt, M. M., Blackshear, C. & Maheshwari, A. Clinical determinants of postoperative outcomes in neonates with surgical necrotizing enterocolitis. J. Perinatol. (2020). in review.
Bowker, R. M., Yan, X. & De Plaen, I. G. Intestinal microcirculation and necrotizing enterocolitis: the vascular endothelial growth factor system. Semin. Fetal Neonatal Med. 23, 411–415 (2018).
Precioso, A. R. & Proenca, R. S. Necrotizing enterocolitis, pathogenesis and the protector effect of prenatal corticosteroids. Rev. Hosp. Clin. Fac. Med. Sao Paulo 57, 243–248 (2002).
Gellen, B. et al. Vascular changes play a role in the pathogenesis of necrotizing enterocolitis in asphyxiated newborn pigs. Pediatr. Surg. Int. 19, 380–384 (2003).
Eaton, S., Sebire, N., Thyoka, M. & Pierro, A. Histologic and immunohistochemical features associated with outcome in neonatal necrotizing enterocolitis. Eur. J. Pediatr. Surg. 24, 51–56 (2014).
Sheng, Q. et al. Short-term surgical outcomes of preterm infants with necrotizing enterocolitis: a single-center experience. Medicine (Baltim.) 95, e4379 (2016).
Wright, N. J. et al. The outcome of critically ill neonates undergoing laparotomy for necrotising enterocolitis in the neonatal intensive care unit: a 10-year review. J. Pediatr. Surg. 49, 1210–1214 (2014).
Li, X., Li, L., Wang, Y., Deng, C. & Guo, C. Postoperative characteristics of infants who developed necrotizing enterocolitis with different postnatal ages. Medicine (Baltim.) 96, e7774 (2017).
Hau, E. M. et al. Gastrointestinal sequelae after surgery for necrotising enterocolitis: a systematic review and meta-analysis. Arch. Dis. Child Fetal Neonatal Ed. 104, F265–F273 (2019).
Geng, Q., Wang, Y., Li, L. & Guo, C. Early postoperative outcomes of surgery for intestinal perforation in NEC based on intestinal location of disease. Medicine (Baltim.) 97, e12234 (2018).
Cole, C. R., Hansen, N. I., Higgins, R. D., Ziegler, T. R., Stoll, B. J. & Eunice Kennedy Shriver, N. N. R. N. Very low birth weight preterm infants with surgical short bowel syndrome: incidence, morbidity and mortality, and growth outcomes at 18 to 22 months. Pediatrics 122, e573–e582 (2008).
Ballance, W. A., Dahms, B. B., Shenker, N. & Kliegman, R. M. Pathology of neonatal necrotizing enterocolitis: a ten-year experience. J. Pediatr. 117, S6–S13 (1990).
Remon, J. I. et al. Depth of bacterial invasion in resected intestinal tissue predicts mortality in surgical necrotizing enterocolitis. J. Perinatol. 35, 755–762 (2015).
Yan, X. et al. Lack of VEGFR2 signaling causes maldevelopment of the intestinal microvasculature and facilitates necrotizing enterocolitis in neonatal mice. Am. J. Physiol. Gastrointest. Liver Physiol. 310, G716–G725 (2016).
Mutanen, A., Pierro, A. & Zani, A. Perioperative complications following surgery for necrotizing enterocolitis. Eur. J. Pediatr. Surg. 28, 148–151 (2018).
Pierro, A. & Hall, N. Surgical treatments of infants with necrotizing enterocolitis. Semin. Neonatol. 8, 223–232 (2003).
Blakely, M. L. et al. Postoperative outcomes of extremely low birth-weight infants with necrotizing enterocolitis or isolated intestinal perforation: a prospective cohort study by the NICHD Neonatal Research Network. Ann. Surg. 241, 984–989 (2005). discussion 989–994.
Jammeh, M. L. et al. Racial/ethnic differences in necrotizing enterocolitis incidence and outcomes in premature very low birth weight infants. J. Perinatol. 38, 1386–1390 (2018).
Stoll, B. J., Eunice Kennedy Shriver National Institute of Child H, Human Development Neonatal Research N. et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993–2012. JAMA 314, 1039–1051 (2015).
Bell, M. J., Feigin, R. D., Ternberg, J. L. & Brotherton, T. Evaluation of gastrointestinal microflora in necrotizing enterocolitis. J. Pediatr. 92, 589–592 (1978).
Gewolb, I. H., Schwalbe, R. S., Taciak, V. L., Harrison, T. S. & Panigrahi, P. Stool microflora in extremely low birthweight infants. Arch. Dis. Child Fetal Neonatal Ed. 80, F167–F173 (1999).
Smith, B. et al. Investigation of the early intestinal microflora in premature infants with/without necrotizing enterocolitis using two different methods. Pediatr. Res. 71, 115–120 (2012).
Watkins, D. J. & Besner, G. E. The role of the intestinal microcirculation in necrotizing enterocolitis. Semin. Pediatr. Surg. 22, 83–87 (2013).
Szabo, J. S., Mayfield, S. R., Oh, W. & Stonestreet, B. S. Postprandial gastrointestinal blood flow and oxygen consumption: effects of hypoxemia in neonatal piglets. Pediatr. Res. 21, 93–98 (1987).
Nowicki, P. T. et al. Intestinal O2 consumption in necrotizing enterocolitis: role of nitric oxide. Pediatr. Res. 59, 500–505 (2006).
Nowicki, P. T., Stonestreet, B. S., Hansen, N. B., Yao, A. C. & Oh, W. Gastrointestinal blood flow and oxygen consumption in awake newborn piglets: effect of feeding. Am. J. Physiol. 245, G697–G702 (1983).
Fernandez, R. et al. Identification of biomarkers of necrosis in xenografts using imaging mass spectrometry. J. Am. Soc. Mass Spectrom. 27, 244–254 (2016).
Tata, A. et al. Rapid detection of necrosis in breast cancer with desorption electrospray ionization mass spectrometry. Sci. Rep. 6, 35374 (2016).
Andrews, W. W. et al. The Alabama Preterm Birth study: polymorphonuclear and mononuclear cell placental infiltrations, other markers of inflammation, and outcomes in 23- to 32-week preterm newborn infants. Am. J. Obstet. Gynecol. 195, 803–808 (2006).
Dorling, J., Kempley, S. & Leaf, A. Feeding growth restricted preterm infants with abnormal antenatal Doppler results. Arch. Dis. Child Fetal Neonatal Ed. 90, F359–F363 (2005).
Venkatesh, K. K. et al. Association of chorioamnionitis and its duration with neonatal morbidity and mortality. J. Perinatol. 39, 673–682 (2019).
Peterslund, P. et al. Frequencies of immune cells in the human small bowel during normal gestation and in necrotizing enterocolitis. Fetal Pediatr. Pathol. 38, 153–166 (2019).
Acknowledgements
We thank Dr. Neelesh Tipnis for helping us in developing and reviewing the manuscript. This research was funded by National Institutes of Health awards HL124078 and HL133022 (to A.M.).
Author information
Authors and Affiliations
Contributions
P.M.G., A.M., and A.G.S. designed the study. P.M.G., A.B., M.M.H., A.K., C.B., and A.G.S. collected and analyzed the data. All the authors contributed to and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Garg, P.M., Bernieh, A., Hitt, M.M. et al. Incomplete resection of necrotic bowel may increase mortality in infants with necrotizing enterocolitis. Pediatr Res 89, 163–170 (2021). https://doi.org/10.1038/s41390-020-0975-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-020-0975-6
This article is cited by
-
Outcomes by disease onset, sex, and intervention in neonates with SIP and surgical NEC
Pediatric Research (2024)
-
Clinical utilization of intestinal pathology in the classification of NEC vs SIP cases and prognostication
Journal of Perinatology (2024)
-
Postoperative Outcomes, and Growth and Brain Injury Outcomes in Spontaneous Intestinal Perforation vs Surgical Necrotizing Enterocolitis in Preterm Infants
Indian Pediatrics (2023)
-
Brain injury in preterm infants with surgical necrotizing enterocolitis: clinical and bowel pathological correlates
Pediatric Research (2022)
-
Early Career Investigator: Biocommentary
Pediatric Research (2021)