Abstract
Background
Perinatal inflammation adversely affects health. Therefore, aims of this IRB-approved study are: (1) compare inflammatory compounds within and between maternal and umbilical cord blood samples at the time of delivery, (2) assess relationships between inflammatory compounds in maternal and cord blood with birth characteristics/outcomes, and (3) assess relationships between blood and placental fat-soluble nutrients with blood levels of individual inflammatory compounds.
Methods
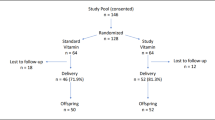
Mother−infant dyads were enrolled (n = 152) for collection of birth data and biological samples of maternal blood, umbilical cord blood, and placental tissue. Nutrient levels included: lutein + zeaxanthin; lycopene; α-, β-carotene; β-cryptoxanthin; retinol; α-, γ-, δ-tocopherol. Inflammatory compounds included: tumor necrosis factor-α, superoxide dismutase, interleukins (IL) 1β, 2, 6, 8, 10.
Results
Median inflammatory compound levels were 1.2–2.3 times higher in cord vs. maternal blood, except IL2 (1.3 times lower). Multiple significant correlations existed between maternal vs. infant inflammatory compounds (range of r = 0.22–0.48). While relationships existed with blood nutrient levels, the most significant were identified in placenta where all nutrients (except δ-tocopherol) exhibited relationships with inflammatory compounds. Relationships between anti-inflammatory nutrients and proinflammatory compounds were primarily inverse.
Conclusion
Inflammation is strongly correlated between mother−infant dyads. Fat-soluble nutrients have relationships with inflammatory compounds, suggesting nutrition is a modifiable factor.
Impact
-
Mother and newborn inflammation status are strongly interrelated.
-
Levels of fat-soluble nutrients in blood, but especially placenta, are associated with blood levels of proinflammatory and anti-inflammatory compounds in both mother and newborn infant.
-
As fat-soluble nutrient levels are associated with blood inflammatory compounds, nutrition is a modifiable factor to modulate inflammation and improve perinatal outcomes.
Similar content being viewed by others
Introduction
Presence of systemic inflammation in adults negatively impacts health outcomes and risk for chronic disease development. However, the presence of inflammation must be considered throughout the life course, especially during fetal development when physical growth is exponential; inflammatory stimuli (i.e. cytokines) are able to cross the blood−brain barrier and any inflammatory-mediated physiological changes carry the risk of lifelong impact.
Past research indicates a strong inverse relationship between levels of proinflammatory compounds during the perinatal period and adverse outcomes in offspring. In example, proinflammatory compounds in either maternal blood or umbilical cord blood have been associated with increased risk of offspring developing cerebral palsy,1,2 depression,3 schizophrenia,4 or psychosis.5 Maternal inflammatory compound levels during pregnancy have also been associated with offspring brain damage6 or white matter alterations7 as identified by magnetic resonance imaging. Inflammatory compound levels may vary in accordance with preterm birth,8 but inflammation in preterm infants has been associated with decreased weight gain and linear growth, decreased developmental outcomes at 18–24 months of age, and an increased risk of failing a newborn hearing screen.9,10,11,12 Mode of infant delivery has also been identified to alter inflammatory compound levels in infants13,14,15 and placental tissue,16 with others concluding no difference.17 Despite evidence highlighting the relationship between perinatal inflammation and offspring outcomes, the relationships between levels of maternal and newborn infant blood inflammatory compounds are not well understood. Furthermore, it is undetermined if mother vs. infant levels of inflammatory compounds have stronger associations with birth outcomes, and how the placenta may mediate these relationships.
There are a multitude of maternal factors that increase fetal inflammatory exposures, including infection, suboptimal nutrition, limited exercise, smoking, chronic disease development (i.e. diabetes), and obesity. Of these, nutrition status remains the most modifiable, dually important for mother and infant, and applicable to all subsets of perinatal populations. Nutritional factors may ameliorate or exacerbate inflammatory stimuli,18 so assessment remains most critical during pregnancy when higher dietary intakes of nutrients are recommended to support fetal growth and ameliorate oxidative stress induced by pregnancy.19,20 Nutrition status is also essential in preterm infants who are at risk for deficiency related to limited accrual of intrauterine nutrients and a subsequent reliance on adequate postnatal dietary supplementation.21 Nutrients of particular interest are fat-soluble vitamins and carotenoids, including retinol (vitamin A), isoforms of vitamin E (α-tocopherol, γ-tocopherol, δ-tocopherol), and lutein, zeaxanthin, lycopene, α-carotene, β-carotene, and β-cryptoxanthin (carotenoids).20 Pregnant women and infants must acquire these compounds through dietary sources as they are unable to be synthesized by humans.20,22,23 Many of these nutrients are primarily obtained through intake of plant-based foods, though others are present in animal-based products.24 Fat-soluble nutrients are theorized to hold anti-inflammatory properties, with the exception of γ-tocopherol which is proinflammatory.22 In contrast, little is known about the inflammatory effects of isoform δ-tocopherol. While nutrition may impact inflammation status, no previous research has compared the relationships between blood or placental concentrations of these fat-soluble nutrients with blood inflammatory compounds during the perinatal period. However, understanding these relationships could have important implications for nutrition interventions that seek to regulate inflammation and enhance birth outcomes.
Therefore, the primary aims of this study were to (1) compare inflammatory compounds within and between maternal and umbilical cord blood samples at time of delivery, (2) assess the relationships between inflammatory compounds in maternal and umbilical cord blood with birth characteristics/outcomes, and (3) assess the relationships between blood and placental fat-soluble nutrients with blood levels of individual inflammatory compounds.
Methods
Subject enrollment
All subjects gave their written informed consent for inclusion before they participated in the study. The study was approved by the Institutional Review Board by the University of Nebraska Medical Center. Mother−infant dyads were enrolled at the time of delivery at Nebraska Medicine Hospital (Omaha, NE, USA) between June and August of 2016–2018. Inclusion criteria included mothers at >19 years of age, delivering at least one live-born infant, and able to make their own medical decisions. Mothers <19 years of age were able to participate with their parental consent. Mother−infant dyads must be free of renal, metabolic, or hepatic diseases that impair normal nutrient metabolism. Preterm infants were included if they met initial criteria. In cases of multiple gestation, data were collected for the first-born infant only. Following enrollment, demographic and clinical data were collected from the electronic medical record.
Data and sample collection
Continuous demographic and outcome variables collected included: maternal age, pre-pregnancy body mass index (BMI) in kilograms/meters2 (kg/m2) based on maternal self-reported height and weight at time of delivery, infant corrected gestational age (CGA) at delivery, birth weight (grams, g) and growth percentile, birth length and head circumference (centimeters, cm) with growth percentile, and infant 1-min and 5-min Apgar score. Maternal BMI was calculated from self-reported measurements as pre-pregnancy measurements were not available for all mothers within the electronic health record. Growth percentiles for infant anthropometric measurements were taken from the World Health Organization 0–2-year growth chart for infants born >37 weeks CGA or the 2013 Fenton growth chart for preterm infants born <37 weeks CGA. Categorical variables collected included: maternal race, maternal smoking status, delivery mode, infant sex, and incidence of maternal obesity (BMI ≥ 30 kg/m2), maternal diabetes during pregnancy (type 1, type 2, or gestational), preeclampsia, infant admission to the newborn intensive care unit (NICU), infant respiratory distress syndrome (RDS), preterm birth (defined as birth <37 weeks gestational age25), and newborn failed hearing screen.
A sample of maternal blood (target volume of 1 mL) was collected as part of a routine blood draw. A sample of umbilical cord blood was collected (target volume of 5 mL), as this is collected at all deliveries and stored in the hospital laboratory for use when needed. A placental tissue sample (taken as a cross-section of the placental disk) was collected at delivery (target weight 10 g). All samples were light protected and frozen immediately at −80 °C once attained by the research team in order to preserve integrity.
Sample analysis
Pro- and anti-inflammatory compound levels were selected for analysis on the basis that they have been previously proposed to correlate with infant neurologic outcomes or have potential neuroprotective anti-inflammatory effects (though primarily in non-infant populations).1,26,27 Selected inflammatory mediator levels were measured via a commercially available multi-analyte bead array (Millipore; Burlington, MA) per the manufacturer’s instructions. This included testing for tumor necrosis factor-α (TNFα), interleukin-1β (IL1β), interleukin-6 (IL6), interleukin-8 (IL8), interleukin-10 (IL10), and interleukin-2 (IL2). Superoxide dismutase (SOD), an endogenous anti-oxidant enzyme that plays a role in the anti-inflammatory effects of some nutritional compounds,28 was measured in maternal and infant samples using the SOD assay kit-WST (WST-1, 2-(4-Iodopheny)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt) from Dojindo (Rockville, MD).29 This assay measures the percent inhibition of WST reduction by SOD, with one unit of SOD representing the amount of enzyme in 20 µL of the sample that results in a 50% reduction of WST-123. Inflammatory compounds are reported in picograms/milliliter (pg/mL).
Serum carotenoid and tocopherol levels were analyzed by the Nutritional Biomarker Lab at the Harvard T. H. Chan School of Public Health using high-performance liquid chromatography (HPLC) as described by El-Sohemy et al.30 Measured levels included that of combined lutein + zeaxanthin, β-cryptoxanthin, total lycopene, total α-carotene, total-β-carotene, retinol, α-tocopherol, δ-tocopherol, and γ-tocopherol. HPLC also measured serum concentrations of α- and γ-tocopherol, but with methods as described by Hanson et al.22 Placenta tissue samples were weighed and homogenized by mechanical pulverization (Polytron PT1200, Kinematica AG; Lucerne, Switzerland), then formed into an aqueous slurry with distilled, deionized water. Samples were mixed with ethanol containing rac-Tocopherol (Tocol) as an internal standard, then lipophilic components were extracted with hexane, evaporated to dryness under nitrogen, and reconstituted in ethanol, dioxane, and acetonitrile. HPLC quantified samples on a Restek Ultra C18 150 mm × 4.6 mm column, 3 μm particle size encased in a Hitachi L-2350 column oven to maintain consistent temperatures and furnished with a trident guard cartridge system (Restek, Corp.; Bellefonte, PA). Acetonitrile, tetrahydrofuran, and methanol were mixed and used as a mobile phase when combined with a 1% ammonium acetate solution (68:22:7:3). A flow rate was set at 1.1 mL/min using a Hitachi Elite LaChrom HPLC system equipped with an L-2130 pump in isocratic mode, an L-2455 Diode Array Detector (monitoring at 300 and 445 nm), and a programmable AS-2200 auto-sampler with chilled sample tray. Peak integration and data acquisition was completed with the system manager software (Hitachi; San Jose, CA). The minimum detection limits in plasma were 3.86 micrograms/Liter (mcg/L) for lutein + zeaxanthin, 3.88 mcg/L for β-cryptoxanthin, 5.44 mcg/L for lycopene, 4.24 mcg/L for α-carotene, and 4.80 mcg/L for β-carotene. Two replicates each of a large-volume two-level plasma pool were included in each sample batch. These four sample controls provided estimates for internal quality control. Quality control was also assessed externally by participation in the standardization program for carotenoid analysis through the National Institute of Standards and Technology, USA.
For the purposes of this analysis, inflammatory compounds and fat-soluble nutrients were categorized as proinflammatory, anti-inflammatory, or unknown/pleiotropic effects as shown in Table 1.
Statistical analysis
Descriptive statistics for continuous variables are reported as median, minimum, maximum, and interquartile range (IQR). Categorical data are presented as counts and proportions. The Mann−Whitney U test was used to compare continuous measures between dichotomous groups. Spearman’s correlation coefficients assessed relationships between continuous variables. Multivariate regression analysis, with log transformation of both nutrient levels and inflammatory measures, was used to predict changes in blood inflammatory compound levels based on fat-soluble nutrient levels in blood or placenta after adjusting for race, delivery mode, maternal smoking, maternal pre-pregnancy BMI, and birth CGA. A p value of ≤0.05 was considered statistically significant.
Results
In total, 152 mother−infant dyads were included in the study and variables were analyzed as available from the electronic medical record. Demographic data are reported in Table 2. Categorical responses are reported in Table 3. Median levels of fat-soluble nutrients in maternal serum, umbilical cord blood, and placenta are reported in Table 4. Median levels of both mother and infant inflammatory compounds are listed in Table 5. As detailed in Tables 2 and 3, the study population included primarily term infants (median CGA of 39 4/7 weeks) without significant prenatal or postnatal complications.
Comparisons within and between maternal and infant inflammatory compounds
While there remain no standardized reference ranges for comparison, all median levels of anti- and proinflammatory compounds in our study were estimated at 1.2–2.3 times higher in umbilical cord compared to maternal blood with the exception of IL2, which was 1.3 times lower in infants compared to mothers (Table 5).
Correlations between individual maternal inflammatory compounds and individual infant compounds are all positive and displayed in Fig. 1. All listed raw r values are significant at p < 0.05, with the exception of the correlation between M-IL2 and M-IL8 (r = 0.22) with p value equivalent to 0.05. Figure 2 displays the significant relationships between maternal and infant inflammatory markers; all list positive r values with p < 0.05. Notably, Fig. 1 demonstrates the highest r values between inflammatory compounds IL6 and IL8 in both mothers and infants (0.90 and 0.80 respectively), as well as some balancing relationships between proinflammatory IL1β and pleiotropic IL2 in mothers (0.75) and infants (0.85). Figure 2 displays similar, albeit weaker, correlations between maternal and infant inflammatory compounds, with positive correlations between maternal and infant proinflammatory IL6 and IL8 and similar balancing with pro- and anti-inflammatory compounds.
Relationships between inflammatory compounds and birth characteristics and outcomes
Characteristics
Maternal pre-pregnancy BMI was inversely correlated with M-SOD (r = −0.46; p = 0.02). Median maternal (M)-IL6 (14.8 vs. 8.9, p = 0.025) and M-IL10 (16.0 vs. 11.5; p = 0.069) were significantly higher in white vs. non-white mothers. There were no significant differences in the median levels of inflammatory compounds between mothers with or without a smoking history.
Median umbilical cord blood (C)-TNFα approached significant differences between male vs. female infants (55.5 vs. 48.3; p = 0.09). No other significant differences were noted in inflammatory compounds with regard to infant sex. Median C-IL1β levels showed trend with a decrease in white infants compared to non-white (8.3 vs. 14.5; p = 0.069).
Outcomes
M-IL8 was significantly lower in moms with diabetes during pregnancy (median 4.6 vs. 8.9; p = 0.03). There were no significant differences in the median levels of inflammatory compounds between vaginal vs. Caesarean deliveries.
Median M-IL2 was higher in cases of infant NICU admission compared to non-admitted infants (10.7 vs. 6.6; p = 0.03). Median C-IL6 and C-IL8 were significantly elevated in infants diagnosed with RDS compared to healthy newborns (10.7 vs. 77.9, p = 0.005; 11.6 vs. 67.5, p = 0.003). Infant 1-min Apgar scores were inversely correlated with M-IL6 (r = −0.23, p = 0.045), C-IL6 (r = −0.21, p = 0.055), and C-IL8 (r = −0.34, p < 0.001), but correlated positively with M-SOD (r = 0.39, p = 0.054). Infant 5-min Apgar scores correlated negatively with C-IL6 (r = −0.27, p = 0.016) and C-IL8 (r = −0.28, p = 0.003), but correlated positively with C-IL10 (r = 0.26, p = 0.020). There were no significant differences in the median levels of inflammatory compounds in term vs. preterm infants or in cases of infant hearing screen failure.
Infant anthropometric measurements at birth revealed relationships with inflammatory compounds. Birth weight was negatively correlated with C-IL1β (r = −0.32, p = 0.004) and C-IL10 (r = −0.19, p = 0.041), as well as birth weight percentile (r = −0.34, p = 0.002; r = 0.21, p = 0.02). M-SOD inversely correlated with birth weight percentile (r = −0.39, p = 0.052) and birth length percentile (r = −0.45, p = 0.027). Birth head circumference size (r = −0.30, p = 0.007) and head circumference percentile (r = −0.21, p = 0.060) was also inversely correlated with M-IL6.
Relationships between fat-soluble nutrients and inflammatory compound levels
Table 6 displays results for multivariate regression analysis to predict how log-transformed nutrient levels impact log-transformed inflammatory compounds after adjusting for race, delivery mode, maternal smoking, maternal pre-pregnancy BMI, and birth CGA. Included were identified significant relationships (p ≤ 0.05) or those that approached significance (p < 0.07). In example interpretation, results predict a 10% increase in (log-transformed) lutein + zeaxanthin in umbilical cord blood would lead to a 3.7% decrease in (log-transformed) M-IL2. While findings were significant among varying nutrient levels in maternal or umbilical cord blood, the strongest and most consistent findings were among nutrient levels in placenta. In fact, all placental nutrient levels except for δ-tocopherol showed significant relationships with inflammatory compounds after adjusting for covariates.
Multiple anti-inflammatory fat-soluble nutrients in blood demonstrate significant inverse relationships with proinflammatory compounds. In maternal serum or umbilical cord blood, these include β-cryptoxanthin, α-carotene, β-carotene, and retinol with mixed effects identified between lutein + zeaxanthin, α-tocopherol, and δ-tocopherol and pleiotropic/unknown inflammatory compounds (IL2). In placenta, inverse relationships exist between anti-inflammatory fat-soluble nutrients (all except α-tocopherol) and proinflammatory blood compounds, primarily M-IL8 and M-IL1β. There are also positive relationships between anti-inflammatory compounds and fat-soluble nutrients, such as placental retinol and α-tocopherol with IL10. However, other relationships remained variable, such as that between anti-inflammatory fat-soluble nutrients and pleiotropic/unknown inflammatory compounds like IL2.
Discussion
In this single-center cohort of mother−infant dyads, we observed several significant correlations within and between maternal and infant blood inflammatory compounds at time of birth. We also identified relationships between inflammatory compounds with birth characteristics/outcomes and fat-soluble nutrient levels. Fat-soluble nutrient levels, primarily those in placental tissue, exhibited significant relationships with blood inflammatory compounds after adjusting for multiple covariates. These integrated results suggest optimizing maternal status of fat-soluble nutrients may favorably alter perinatal inflammation and result in enhanced infant birth outcomes.
Inflammatory compounds
Assessment of perinatal inflammatory status remains highly complex, yet strongly interrelated, as demonstrated by the multiple relationships between maternal and infant inflammatory compounds in Figs. 1 and 2. Notably, all relationships demonstrate weak to moderate positive correlations, concluding that an increase in any singular inflammatory compound demonstrates a ripple effect with increasing additional singular pro- or anti-inflammatory compounds in mothers and infants. Individual inflammatory compound levels showed similar significant relationships between both maternal and infant samples for TNFα, IL1β, IL2, and IL10, which were all interrelated. Likewise, both maternal and infant samples showed significant relationships between IL6 with IL8 and IL2 with IL10.
Birth outcomes
Achieving an optimal balance of pro- and anti-inflammatory mediators may have a significant impact on neonatal outcomes. High levels of maternal + inflammatory compounds such as IL6 have been associated with increased preterm birth.31 Though we found no difference in inflammatory compounds between infants born term vs. preterm, we did identify infants diagnosed with RDS to have higher median levels of IL6 and IL8 in umbilical cord blood. This mimics prior research, as An et al. reported these inflammatory compounds to be elevated in the umbilical cord blood of preterm infants who developed chronic lung disease (defined as need for oxygen supplementation at 28 days of life) vs. those who did not.32 Prior research also indicates extrauterine growth is altered in preterm infants exposed to increased inflammatory stimuli, but our results demonstrate that intrauterine inflammatory exposure also impacts fetal growth and resulting birth anthropometric outcomes. This altered fetal growth may impact metabolic programming, contributing to long-term health consequences as a result of developmental origins of health and disease. We also identified significant inverse relationships between M-IL6 and infant birth head circumference size. Future research is indicated to determine if this is a preliminary finding for risk of altered cognition, as perinatal exposure to IL6 has previously been associated with altered cognitive outcomes.1,7
Nutrient levels
Many identified relationships indicate fat-soluble nutrients mediate inflammation. Multiple anti-inflammatory fat-soluble nutrients in blood (β-cryptoxanthin, α-carotene, β-carotene, and retinol) demonstrate significant inverse relationships with proinflammatory compounds. The strongest of these was between retinol levels in umbilical cord blood and C-IL8, signifying a protective effect for infants. Yet, mixed effects were identified between anti-inflammatory lutein + zeaxanthin, α-tocopherol, and δ-tocopherol with IL2, which is deemed a pleiotropic/unknown inflammatory compound. However, among relationships that remained significant, results would predict a 10% increase in a singular (log-transformed) fat-soluble nutrient to result in a 1.6–9.5% increase or decrease in singular (log-transformed) blood inflammatory cytokine. While these data identified associations between inflammatory and fat-soluble nutrient levels, no causation can be determined. It remains unknown if varying nutrient levels influence inflammatory compound production or if presence of inflammation impacts nutrient transfer from mother to infant.
The most unique relationships between inflammatory compounds and fat-soluble nutrient levels were identified in the placenta. In fact, all fat-soluble nutrients (with the exception of δ-tocopherol) showed significant relationships with inflammatory compounds. Not only did the placenta show the most significant relationships, but also the strongest. Among significant findings, a 10% increase in a singular (log-transformed) fat-soluble nutrient would predict between a 2.5 and 9.2% increase or decrease in a singular (log-transformed) blood inflammatory cytokine. Placental β-cryptoxanthin, lycopene, and α-tocopherol exhibited the most relationships with inflammatory compounds, followed by retinol. Inverse relationships exist between anti-inflammatory fat-soluble nutrients (all except α-tocopherol) and proinflammatory blood compounds. There were also positive relationships between anti-inflammatory fat-soluble nutrients and anti-inflammatory compounds, such as placental retinol and α-tocopherol with IL10. However, there were also some conflicting results in which the relationships between pro- and anti-inflammatory compounds were not as clearly aligned, reflecting the complexity of immune responses. In example, γ-tocopherol, commonly considered a proinflammatory compound, showed an inverse relationship with proinflammatory M-IL8. It may be considered if fat-soluble nutrients express different oxidative properties dependent on the presence in blood or tissue. Alternatively, results suggest the placenta is the most protective defense for the developing fetus, blunting fetal inflammatory exposure at its own expense. Therefore, future research is indicated to further analyze the complexity of these associations and to better understand how these individual responses impact nutrient transfer and resulting perinatal outcomes. Nonetheless, these results show a promising potential benefit of optimizing perinatal nutrition.
As infant growth, neurodevelopment, and birth outcomes are negatively impacted by inflammation, modifiable lifestyle factors that have potential to blunt adverse effects must be considered. As maternal blood levels of carotenoids are strongly related to dietary intake of fruits and vegetables33 and optimizing nutrition is a safe intervention with many other health benefits, this remains an important target of both individual prenatal nutrition counseling as well as large-scale programmatic strategies for optimizing perinatal nutrition. Additional research is also needed to identify long-term outcomes related to inflammatory compound levels at the time of birth and if threshold values exist for fat-soluble nutrient levels that favorably modulate inflammation to support improved perinatal health outcomes.
Strengths and limitations
The unique strengths of this study are that we assessed relationships of multiple complex inflammatory compounds within a diverse population of mother−infant dyads, which has been a gap in prior research. Furthermore, we assessed nutrient status in three primary biological samples that have the potential to impact inflammation status. The intricate analyses conducted significantly enhance our understanding of the interplay between perinatal inflammation status, fat-soluble nutrient concentrations, and resulting birth outcomes. This work provides baseline for future interventional studies that seek to optimize perinatal nutrition as a method to modulate inflammation and enhance birth outcomes.
The weakness of this study is that results are cross-sectional and do not allow for comparison of inflammation or nutrition status at the time of conception, during pregnancy, or postpartum. While we adjusted for multiple covariates, we acknowledge that additional factors may impact inflammation status. Though our cohort was diverse and included dyads with pregnancy complications like diabetes or preterm birth, a majority of mother−infant dyads delivered at term gestational ages and were relatively free of complications. Therefore, our results are not reflective of inflammatory responses in pathological pregnancies (i.e. chorioamnionitis, fetal inflammatory response syndrome, etc.), which are likely to differ substantially from nonpathologic pregnancies.
Conclusions
This study demonstrates that there are significant correlations between maternal and umbilical cord blood levels of inflammatory compounds. Carotenoid, retinol, and tocopherol levels in blood and placenta show relationships with inflammatory compound levels in maternal or umbilical cord blood, suggesting nutrition is a modifiable factor influencing perinatal inflammation status. Additional research is needed to identify long-term outcomes related to inflammatory compound levels at the time of birth and threshold fat-soluble nutrient values that may favorably modulate inflammation to support improved perinatal health outcomes.
References
Armstrong-Wells, J. et al. Inflammatory predictors of neurologic disability after preterm premature rupture of membranes. Am. J. Obstet. Gynecol. 212(Feb), 212.e1–212.e9 (2015).
Bi, D. et al. The association between sex-related interleukin-6 gene polymorphisms and the risk for cerebral palsy. J. Neuroinflammation. 11(Jun), 100 (2014).
Gilman, S. E. et al. Prenatal immune programming of the sex-dependent risk for major depression. Transl. Psychiatry 6(May), e822 (2016).
Brown, A. S. et al. Elevated maternal interleukin-8 levels and risk of schizophrenia in adult offspring. Am. J. Psychiatry 161(May), 889–895 (2004).
Buka, S. L. et al. Maternal cytokine levels during pregnancy and adult psychosis. Brain Behav. Immun. 15(Dec), 411–420 (2001).
Tian, C., Cheng, L. & Gu, X. Cord blood TNF-alpha and IL-6 levels as diagnostic indicators of brain damage in neonates with non-asphyxia fetal distress. Arch. Gynecol. Obstet. 295(Feb), 337–342 (2017).
Rasmussen, J. M. et al. Maternal interleukin-6 concentration during pregnancy is associated with variation in frontolimbic white matter and cognitive development in early life. Neuroimage 185(Jan), 825–835 (2019).
Blanco-Quiros, A. et al. Cord blood interleukin-10 levels are increased in preterm newborns. Eur. J. Pediatr. 159(Jun), 420–423 (2000).
Rose, J., Vassar, R., Cahill-Rowley, K., Hintz, S. R. & Stevenson, D. K. Neonatal biomarkers of inflammation: correlates of early neurodevelopment and gait in very-low-birth-weight preterm children. Am. J. Perinatol. 33(Jan), 71–78 (2016).
Cuestas, E., Aguilera, B., Cerutti, M. & Rizzotti, A. Sustained neonatal inflammation is associated with poor growth in infants born very preterm during the first year of life. J. Pediatr. 205(Feb), 91–97 (2019).
Ramel, S. E. et al. The relationship of poor linear growth velocity with neonatal illness and two-year neurodevelopment in preterm infants. Neonatology 102, 19–24 (2012).
Shim, Y. J. et al. Inflammatory and immune proteins in umbilical cord blood: association with hearing screening test failure in preterm neonates. Mediators Inflamm. 2018(Sep), 4209359 (2018).
Trevino-Garza, C. et al. Leptin, IL-6 and TNF-alpha levels in umbilical cord blood of healthy term newborns in relation to mode of delivery. J. Obstet. Gynaecol. 36(Aug), 719–721 (2016).
Gedikbasi, A. et al. The evaluation of cord blood interleukin-1beta levels in normal and caesarean deliveries. Hum. Exp. Toxicol. 33(Dec), 1193–1198 (2014).
Wampach, L. et al. Birth mode is associated with earliest strain-conferred gut microbiome functions and immunostimulatory potential. Nat. Commun. 30(Nov), 5091 (2018).
Hu, Y. et al. Placenta response of inflammation and oxidative stress in low-risk term childbirth: the implication of delivery mode. BMC Pregnancy Childbirth 17(Dec), 407 (2017).
Tutdibi, E., Hunecke, A., Lindner, U., Monz, D. & Gortner, L. Levels of cytokines in umbilical cord blood in relation to spontaneous term labor. J. Perinat. Med. 40(Sep), 527–532 (2012).
Cusick, S. E. & Georgieff, M. K. The Role of Nutrition in Brain Development: The Golden Opportunity of the “First 1000 Days”. J Pediatr. 175, 16–21. https://doi.org/10.1016/j.jpeds.2016.05.013 (2016).
Institute of Medicine (US) Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc (National Academy of Sciences, Washington, DC, 2001).
Institute of Medicine (US) Panel on Dietary Antioxidants and Related Compounds. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids (National Academies Press, Washington, DC, 2000).
Shah, M. D. & Shah, S. R. Nutrient deficiencies in the premature infant. Pediatr. Clin. North Am. 56(Oct), 1069–1083 (2009).
Hanson, C., Lyden, E., Furtado, J., Van Ormer, M. & Anderson-Berry, A. A. Comparison of nutritional antioxidant content in breast milk, donor milk, and infant formulas. Nutrients 8(Oct), E681 (2016).
Ozsurekci, Y. & Aykac, K. Oxidative stress related diseases in newborns. Oxid. Med. Cell Longev. 2016, 2768365 (2016).
United States Department of Agriculture. USDA Food Composition Databases https://ndb.nal.usda.gov/ndb/nutrients/. Accessed July 31, 2020.
World Health Organization. Preterm Birth. 2018. https://www.who.int/news-room/fact-sheets/detail/preterm-birth. Accessed July 31, 2020.
Zhou, Z. W. et al. Erythropoietin regulates immune/inflammatory reaction and improves neurological function outcomes in traumatic brain injury. Brain Behav. 7(Oct), e00827 (2017).
Machova Urdzikova, L. et al. A green tea polyphenol epigallocatechin-3-gallate enhances neuroregeneration after spinal cord injury by altering levels of inflammatory cytokines. Neuropharmacology 126(Nov), 213–223 (2017).
Yahfoufi, N., Alsadi, N., Jambi, M., & Matar, C. The Immunomodulatory and anti-inflammatory role of polyphenols. Nutrients https://doi.org/10.3390/nu10111618 (2018).
Dojindo Molecular Technologies, I. SOD Assay Kit-WST. https://www.dojindo.com/product/sod-assay-kit-wst-s311/. Accessed November 30, 2020.
El-Sohemy, A. et al. Individual carotenoid concentrations in adipose tissue and plasma as biomarkers of dietary intake. Am. J. Clin. Nutr. 76(Jul), 172–179 (2002).
Sorokin, Y. et al. Maternal serum interleukin-6, C-reactive protein, and matrix metalloproteinase-9 concentrations as risk factors for preterm birth. Am J Neonatol. 8(Sept), 631–640 (2010). https://doi.org/10.1055/s-0030-1249366.
An, H. et al. Interleukin-6, interleukin-8, and soluble tumor necrosis factor receptor-I in the cord blood as predictors of chronic lung disease in premature infants. Am. J. Obstet. Gynecol. 191(Nov), 1649–1654 (2004).
Zielinska, M. A., Wesolowska, A., Pawlus, B. & Hamulka, J. Health Effects of carotenoids during pregnancy and lactation. Nutrients https://doi.org/10.3390/nu9080838 (2017).
Acknowledgements
Carotenoid analysis was funded by the Child Health Research Institute by Children’s Hospital & Medical Center and the University of Nebraska Medical Center. Inflammatory mediator analysis was funded by the University of Nebraska Medical Center Clinical Translational Research Mentored Scholars Program Pilot Award to J.N.S.
Author information
Authors and Affiliations
Contributions
Conceptualization, all authors; methodology, all authors; software, all authors; validation, all authors; formal analysis, E.R.L.; investigation, all authors; resources, all authors; data curation, all authors; investigation, all authors; writing—original draft preparation, M.K.T. and J.N.S.; writing—review and editing, all authors; visualization, all authors; supervision; A.L.A.-B., C.K.H. and J.N.S.; project administration, J.N.S.; funding acquisition, M.K.T., A.L.A.-B., C.K.H. and J.N.S.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent statement
Written informed consent was obtained for each mother−infant dyad before enrollment into this study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Thoene, M.K., Van Ormer, M.C., Lyden, E.R. et al. Concentrations of fat-soluble nutrients and blood inflammatory compounds in mother−infant dyads at birth. Pediatr Res 90, 436–443 (2021). https://doi.org/10.1038/s41390-020-01302-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-020-01302-8