Abstract
Background
Early identification of pregnant women at risk of delivering small-for-gestational-age (SGA) infants is required to reduce the rates of mortality and morbidity in their whole life. This meta-analysis was performed to determine whether women with higher blood alpha-fetoprotein (AFP) levels are at increased risk of SGA.
Methods
Studies identified by searching 11 databases, including PubMed, were assessed using the Newcastle–Ottawa Scale. Subgroup and meta-regression analyses and sensitivity analysis removing a potential outlier were performed. Publication bias was assessed using Egger’s test.
Results
A total of 39 good-quality cohort studies involving 93,968 women and their newborn infants or fetuses ensured both internal and external validity. Relative risk of SGA among women with higher in comparison to lower blood AFP levels was 2.021 (95% CI: 1.751–2.334). Maternal blood AFP levels showed a dose–response relationship with risk of SGA. Relative risk was higher with diagnosis of SGA by ultrasound than actual birth weight (P = 0.004). Sources of heterogeneity, subgroups, confounding factors, or potential outliers did not alter the interpretations without publication bias (P = 0.320).
Conclusions
Pregnant women with higher blood AFP levels are at increased risk of SGA compared to those with lower blood AFP levels.
Impact
-
Pregnant women with higher blood alpha-fetoprotein levels (AFP) levels are at increased risk of small for gestational age (SGA) compared to those with lower blood AFP levels.
-
High-quality evidence showed a dose–response relationship of maternal blood AFP levels with risk of delivering SGA and was robust to sources of heterogeneity, subgroups, confounding factors, potential outliers, or publication bias.
-
Politically and practically, monitoring of maternal blood AFP level is strongly recommended to identify women at risk of delivering SGA.
Similar content being viewed by others

Introduction
The rates of mortality and morbidity in both the perinatal period and later in life are higher among small for gestational age (SGA) infants (defined typically as birth weight <10th percentile for gestational age [and sex]) than their non-SGA counterparts.1,2,3,4 Thus it is necessary to identify women at risk of delivering SGA babies early during pregnancy to establish suitable preconceptional and periconceptional strategies to reduce the rates of mortality and morbidity in both of these periods. There is no evidence that maternal anthropometry, including height, weight, and body mass index (BMI), can be used for diagnosis of SGA and low birth weight (birth weight <2500 g).5,6,7 However, two dose–response meta-analyses including a total of 14 and 10 high-quality studies concluded that there were inverse associations of maternal height and weight and maternal BMI, respectively, with risk of delivering SGA infants.8,9 On the other hand, adiponectin and leptin are biologically active molecules secreted by adipose tissue, both of which play important roles in fetal growth.10,11,12,13 However, two other meta-analyses including a total of 8 and 32 (overall) good-quality studies yielded no rigorous evidence of significant differences in maternal blood adiponectin and leptin levels, respectively, between women delivering SGA and healthy controls.10,11
SGA with pathological growth restriction is caused by placental insufficiency,14,15,16 although there is another subgroup of SGA consisting of healthy fetuses without placental insufficiency that are constitutionally small.16 Maternal blood biomarkers currently used to screen for Down’s syndrome, including alpha-fetoprotein (AFP), beta-human chorionic gonadotropin (beta-hCG), and pregnancy-associated plasma protein A (PAPP-A), are possible indicators of placental dysfunction.17,18,19 In this connection, synthetic evidence based on a previous meta-regression analysis including a total of 81 relatively good-quality studies showed positive associations of high maternal blood AFP and beta-hCG levels and low maternal blood PAPP-A level with the risk of low birth weight.19 This meta-analysis including cohort and case–control studies was performed to determine whether pregnant women with higher blood AFP levels are at increased risk of delivering SGA infants compared to those with lower blood AFP levels.
Methods
Primary outcomes and eligibility criteria
The primary outcomes were relative risk of delivering SGA babies among pregnant women with higher in comparison to lower blood AFP levels. The eligibility criteria were: (a) English language studies, (b) no restrictions on the range of publication years, (b) cohort or case–control design, (c) involving pregnant women with singleton pregnancies, (d) SGA defined as birth weight <10th percentile according to gestational age (and sex), and (e) providing the number of SGA infants and the number of non-SGA infants among women with higher blood AFP levels and the number of SGA infants and the number of non-SGA infants among those with lower blood AFP levels to fill 2 × 2 tables.
Search strategy, electronic databases, and study selection
PubMed (MEDLINE) was searched between March 2019 and August 2019 with no restrictions on the range of publication years. The search terms are described in Supplementary Methods. Articles determined to be unrelated to the purpose of the analysis by scanning their titles and abstracts were excluded, and the remaining articles were selected. Articles determined to be unrelated to the purpose of the analysis by reviewing their full texts were also excluded. The remaining articles became potentially eligible articles. Articles reporting studies that did not satisfy the eligibility criteria and both reviews and letters to the editor were excluded. The remaining articles became eligible for inclusion in the final analysis. The articles provided by clicking “See all…” displayed on the columns on the right side of the screens showing the titles and abstracts of potentially eligible articles and the articles provided in the reference sections of potentially eligible articles were also investigated. Ten other electronic databases described in Supplementary Methods were searched to identify additional articles. The selection process was repeated periodically.
Data extraction and study quality assessment
The characteristics of the included studies and the numbers of SGA infants and the numbers of non-SGA infants among pregnant women with higher blood AFP levels and the numbers of SGA infants and the numbers of non-SGA infants among those with lower blood AFP levels were extracted to fill 2 × 2 tables. The characteristics of the included studies were first author, publication year, study countries, exclusion criteria, study design, data collection, responses to nine questions of the Newcastle–Ottawa Scale (NOS) to assess the quality of nonrandomized studies in meta-analyses (NOS),20 NOS score (defined as number of “Yes” responses to nine questions in the NOS), assay methods, cutoff points, periods of AFP test, and methods used for diagnosis of SGA. Duplicated data were integrated. The most frequent responses to nine questions of the NOS20 determined after five assessments were selected as final responses. This quality assessment was used to evaluate whether the findings were subject to serious bias (internal validity). The total number of included studies, the total number of women and their neonates, and study regions were used to evaluate whether the findings were generalizable (external validity).
Study region was categorized into Africa, Asia, Europe, Latin America, the Middle East, North America, and Oceania and developing and developed countries. Exclusion criteria were categorized into congenital malformation and preeclampsia. Study design and data collection were categorized into cohort and case–control studies and prospective and retrospective data collection, respectively. Study quality assessment was categorized into “Yes”, “Unclear”, and “No” responses to nine questions of the NOS and NOS score <7 or ≥7. Assay methods, cutoff point, and period of AFP test were categorized into chemiluminescence immunoassay (CLIA), dissociated-enhanced lanthanide fluorescence immunoassay (DELFIA), or automated DELFIA (AutoDELFIA), enzyme immunoassay (EIA), enhanced luminescence immunoenzymometric assay (ELIA), and radioimmunoassay (RIA); 1 multiple of median (MoM), 2 MoM, 2.5 MoM, and 3 MoM; and second trimester only and first–second or first–third trimesters, respectively. The method used for diagnosis of SGA was categorized into ultrasound and actual birth weight.
Evidence synthesis
Statistical analyses were performed with Stata/MP 13.1 (StataCorp LP, College Station, TX, USA) (August 2019). Homogeneity and heterogeneity were defined as I2 < 50% and ≥50%, respectively. Investigation of heterogeneity sources was performed to determine whether heterogeneity was altered to homogeneity by selecting studies limited to each of the characteristics of the included studies (see “Data extraction and study quality assessment”), as long as the categorization included two or more studies.
A meta-analysis was performed to pool relative risks of delivering SGA infants among pregnant women with higher in comparison to lower blood AFP levels. A fixed-effects model (i.e., inverse variance method) and a random-effects model (i.e., the DerSimonian and Laird method) were used to pool homogeneous data and heterogeneous data, respectively. The forest plot was displayed to show the relative risk of each study and pooled ones. Subgroup analysis was performed by limiting studies based on the characteristics of included studies in the same way as described for investigation of heterogeneity sources. A value of “0” was assigned to zero cells, and meta-regression analysis was performed to determine statistical significance of differences in the relative risks of delivering SGA among pregnant women with higher in comparison to lower blood AFP levels between a certain category used in investigation of heterogeneity sources and subgroup analysis and its counterpart. Sensitivity analysis was performed to evaluate whether the results were altered after removing a study that was considered to be a potential outlier in the forest plot. Assessment of publication bias was performed using Egger’s test.21
Results
Search results
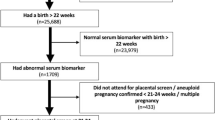
Fourteen articles were identified by searching PubMed (MEDLINE) (Fig. 1). Two articles determined to be unrelated to the purpose of the analysis by scanning the titles and abstracts were excluded. As a result, 12 articles were selected. Fifty-five and 15 articles were identified using PubMed-related citations of potentially eligible articles and the bibliographic references of potentially eligible articles, respectively. One article was also identified by searching using ten other electronic databases. Thus 87 articles were subjected to full-text retrieval. Thirty-four articles determined to be unrelated to the purpose of the analysis were excluded. As a result, 53 articles were classified as potentially eligible. Four and 15 articles which reported studies that did not define SGA as birth weight <10th percentile according to gestational age (and sex) and in which the data for 2 × 2 tables could not be extracted, respectively, were excluded. Six articles determined to be unrelated to the purpose of the analysis were excluded. As a result, 27 articles were finally selected for statistical analysis (Table 1 and Fig. 1).22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48
Extraction of studies
When a single article provided data based on more than one cutoff point of maternal blood AFP, the same number of studies as these cutoff points were extracted from one article. Thus the articles by Cho et al., Di et al., Pandey et al., Puntachai et al., Rodock et al., Secher et al., and Wenstrom et al. reported three, two, two, two, two, four, and four studies, respectively (Table 1).28,29,38,39,40,42,45 As a result, a total of 39 studies involving 93,968 women and their newborn infants or fetuses were extracted to evaluate whether pregnant women with higher blood AFP levels are at increased risk of delivering SGA infants compared to those with lower blood AFP levels (Table 1 and Fig. 2).22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48
Description of studies
Studies were performed in 13 developing and developed countries in Asia, Europe, the Middle East, North America, and Oceania (Table 1). Fourteen studies in ten articles23,24,25,28,29,33,34,35,39,48 and one study in one article36 excluded women with congenital malformations and those with preeclampsia, respectively. All of the studies had a cohort design.22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48 Eleven studies in six articles28,34,35,43,45,48 and five studies in four articles31,33,37,39 collected the data prospectively and retrospectively, respectively. Overall, the studies had good quality, because all of the included studies had NOS score ≥5 (Table 1), and there were a greater number of “Yes” and “Unclear” responses than “No” responses to nine questions of the NOS (Supplementary Fig. 1).22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48 Maternal blood AFP levels were determined using CLIA, DELFIA, AutoDELFIA, ELIA, EIA, and RIA in 2 studies described in 2 articles,24,30 2 studies described in 1 article,39 1 study described in 1 article,48 1 study described in 1 article,23 4 studies described in 3 articles,37,38,47 and 18 studies described in 12 articles,22,28,29,31,32,36,40,41,42,43,44,46 respectively. Cutoff points for diagnosis of SGA varied markedly among studies,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48 but 2 MoM of blood AFP levels was used the most frequently (n = 17).22,24,25,26,27,29,30,31,33,35,36,39,42,43,44,47,48 Thirty-four studies described in 24 articles22,23,24,25,26,27,28,29,31,32,33,34,35,36,37,40,41,42,43,44,45,46,47,48 and 5 studies described in 3 articles30,38,40 took maternal blood AFP level at the second trimester only and at the first–second or the first–third trimesters, respectively. Two studies described in 2 articles22,24 and 37 studies described in 25 articles23,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48 used ultrasound and actual birth weight, respectively, for diagnosis of SGA.
Results of synthesis
In the total population, the data were heterogeneous (Table 2). Heterogeneity was altered to homogeneity by selecting studies limited to Asia, North America, retrospective data collection, DELFIA/AutoDELFIA, cutoff point of 1 MoM, cutoff point of 3 MoM, and SGA diagnosed by ultrasound (investigation of heterogeneity sources). Relative risk of delivering SGA infants among pregnant women with higher in comparison to lower blood AFP levels was >1 (Table 2 and Fig. 2). This was the case in all subgroups subjected to analysis, regardless of I2 (i.e., heterogeneity or homogeneity) and P value (i.e., confounding or non-confounding factors) (Table 2; subgroup analysis). Two confounding factors were identified, i.e., AFP test at second trimester only vs. at first–second or first–third trimesters and SGA diagnosed by actual birth weight vs. ultrasound (P = 0.038 and 0.004, respectively; meta-regression analysis). Even after removing a potential outlier, i.e., the study by Akinbiyi22 (Fig. 2), relative risk of delivering SGA infants among pregnant women with higher in comparison to lower blood AFP levels was >1 (1.978, 95% confidence interval (CI): 1.718–2.276; sensitivity analysis). Egger’s test did not identify publication bias (P = 0.320; publication bias assessment).
Discussion
Principle findings
To the author’s knowledge, this study is the first meta-analysis to evaluate whether pregnant women with higher blood AFP levels are at increased risk of delivering SGA infants compared to those with lower blood AFP levels. The synthetic evidence of the present study suggested that pregnant women with higher blood AFP levels are at increased risk of delivering SGA infants compared to those with lower blood AFP levels (Table 2 and Fig. 2). The synthetic evidence was epidemiologically valid as follows. The findings were well generalizable (external validity) based on a total of 39 studies involving 93,968 women and their newborn infants or fetuses in 13 developing and developed countries in Asia, Europe, the Middle East, North America, and Oceania (Table 1).22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48 The findings were not subject to serious bias (internal validity) based on NOS score ≥5 for all studies (Table 1) and a greater number of “Yes” and “Unclear” responses to nine questions of the NOS than “No” responses (Supplementary Fig. 1). On the other hand, the principal findings provided political, practical, and clinical significance that were relevant to women of childbearing age and their families, healthcare providers, and policy makers. Maternal blood AFP levels can be used to identify women at risk of delivering SGA babies early during pregnancy, which helps in the development of preconceptional and periconceptional strategies to reduce the rates of mortality and morbidity in both the perinatal period and later in life.
Quality of evidence
The following findings indicated that high-quality evidence was provided on the association of high maternal blood AFP levels with risk of delivering SGA infants, based on the Grading of Recommendations, Assessment, Development and Evaluation.49 This meta-analysis included observational studies, and the data in the total population were heterogeneous. However, the association was confirmed in all subgroups subject to heterogeneity or homogeneity, in all those subject to confounding or non-confounding (Table 2), and based on the exclusion of a potential outlier, and no publication bias was detected. That is, the evidence was robust to sources of heterogeneity, subgroups, confounding factors, potential outliers, and publication bias. The exclusion of even two or three studies also had little effect on the results, because the range of the weights of individual studies included in the analysis was 0.26–4.63%.
There were methodologically problematic issues in studies showing that relative risk of delivering SGA infants among pregnant women with higher in comparison to lower blood AFP levels was ≪1 (Table 1 and Fig. 2), as follows. The study (b) by Cho et al. and the study (b) by Rodock et al. used Down’s syndrome risk = 1:270, i.e., 0.80 MoM and 5th percentile (<1 MoM), respectively, as a cutoff point for the AFP test.28,40 Thus the association of high maternal blood AFP levels with risk of delivering SGA infants would have been shown to be stronger with use of a cutoff point >1 MoM (i.e., “confounding is expected to reduce a demonstrated effect”49). The studies (a) and (b) by Pandey et al. used controls limited to maternal blood AFP levels between 0.5 MoM and 2.5 MoM.38 The study by Smith et al. involved newborn infants limited to birth weight <2.5 or >3.0 kg.43
Relative risk of delivering SGA infants among pregnant women with higher in comparison to lower blood AFP levels may have increased by increasing the cutoff point of the AFP test to diagnose SGA from 1 MoM to 3 MoM (Table 2). Relative risk was also substantially higher using the cutoff point ≥3 MoM than <1 MoM (P = 0.010). This suggested a dose–response relationship between maternal blood AFP levels and risk of delivering SGA infants.
AFP test at second trimester only vs. at first–second or first–third trimesters was a confounding factor. As mentioned above, however, there were methodologically problematic issues in all but one study in which maternal blood AFP levels were taken at first–second or first–thrd trimesters, i.e., the studies (a) and (b) by Pandey et al. and the studies (a) and (b) by Rodock et al.38,40 Thus this effect may have been due to the inclusion or exclusion of these studies rather than differences in periods at which maternal blood AFP levels were taken.
SGA diagnosed by actual birth weight vs. ultrasound was another confounding factor. Relative risk of delivering SGA infants among pregnant women with higher in comparison to lower blood AFP levels was substantially higher using ultrasound to diagnose SGA than actual birth weight. This was plausible, because the period between AFP test and SGA diagnosis was shorter when ultrasound was used than actual birth weight. In addition, the magnitude of the effect was large (relative risk > 2) (Table 2 and Fig. 2), based on the definition of large and very large effects.49
Strengths and limitations of study
The first strength of this study was the inclusion of procedures that were consistent overall with the guidance to conduct meta-analyses,50 i.e., identifying a question, designing a study, selecting and reviewing studies, extracting and analyzing data, and interpreting the results. The second strength was external and internal validities of the evidence based on the inclusion of a total of 39 good-quality studies with 93,968 women and their newborn infants or fetuses. The third strength was the high quality of the evidence due to robustness of the interpretation to sources of heterogeneity, confounding factors, publication bias, or potential outliers and due to a dose–response relationship of maternal blood AFP with risk of SGA and magnitude of an effect.
The first limitation was the involvement of only a single person in searches, screening, selection, and review of studies and the exclusion of studies in non-English languages due to a lack of additional reviewer(s) who could use English or non-English language(s). However, PubMed-related citations and bibliographic references were investigated, and the process of study selection was repeated periodically to prevent missing studies that would be finally eligible for the analysis. The second limitation was the magnitude of an effect that was sufficient to identify women at risk of delivering SGA but not sufficient to either exclusively or confirmatively diagnose SGA (e.g., sensitivity and specificity = 0.309, 95% CI: 0.200–0.445 and 0836, 95% CI: 0.736–0.902, respectively). The third limitation was that there was no guarantee that the results of the present study would be applicable to groups not included in the subgroup analysis.
Conclusions and political–practical implications
Maternal blood AFP levels used in Down’s syndrome screening may have another role in preconceptional and periconceptional strategies. The synthetic evidence suggested that pregnant women with higher blood AFP levels (especially >1 MoM) are at increased risk of delivering SGA infants compared to those with lower blood AFP levels. This was compatible with previous evidence on the association of high maternal blood AFP levels with risk of low birth weight infants.19 The evidence in this study was also epidemiologically valid and of high quality. Thus monitoring of maternal blood AFP levels is strongly recommended to identify women at risk of delivering SGA babies, which may help to reduce the rates of mortality and morbidity in both the perinatal period and later in life.
References
Tsai, L. Y., Chen, Y. L., Tsou, K. I. & Mu, S. C. Taiwan Premature Infant Developmental Collaborative Study Group. The impact of small-for-gestational-age on neonatal outcome among very-low-birth-weight infants. Pediatr. Neonatol. 56, 101–107 (2015).
Sharma, P., McKay, K., Rosenkrantz, T. S. & Hussain, N. Comparisons of mortality and pre-discharge respiratory outcomes in small-for-gestational-age and appropriate-for-gestational-age premature infants. BMC Pediatr. 4, 9 (2004).
Giapros, V., Drougia, A., Krallis, N., Theocharis, P. & Andronikou, S. Morbidity and mortality patterns in small-for-gestational age infants born preterm. J. Matern. Fetal Neonatal Med. 25, 153–157 (2012).
Romero, R. The child is the father of the man. Prenat. Neonatal Med. 1, 8–11 (1996).
Goto, E. Maternal anthropometry to predict small for gestational age: a meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 203, 193–198 (2016).
Goto, E. Diagnostic value of maternal anthropometric measurements for predicting low birth weight in developing countries: a meta-analysis. Asia. Pac. J. Clin. Nutr. 24, 260–272 (2015).
Goto, E. Maternal anthropometric measurements as predictors of low birth weight in developing and developed countries. Arch. Gynecol. Obstet. 292, 829–842 (2015).
Goto, E. Dose-response associations of maternal height and weight with small for gestational age: a meta-analysis. Eur. J. Clin. Nutr. 74, 106–111 (2020).
Goto, E. Dose-response association between maternal body mass index and small for gestational age: a meta-analysis. J. Matern. Fetal Neonatal Med. 30, 213–218 (2017).
Goto, E. Maternal and cord blood adiponectin concentrations in small for gestational age: a meta-analysis. Ann. Nutr. Metab. 72, 57–64 (2018).
Goto, E. Maternal blood leptin concentration in small for gestational age: a meta-analysis. Eur. J. Pediatr. 178, 763–770 (2019).
Jaquet, D., Deghmoun, S., Chevenne, D., Czernichow, P. & Lévy-Marchal, C. Low serum adiponectin levels in subjects born small for gestational age: impact on insulin sensitivity. Int. J. Obes. 30, 83–87 (2006).
Briffa, J. F., McAinch, A. J., Romano, T., Wlodek, M. E. & Hryciw, D. H. Leptin in pregnancy and development: a contributor to adulthood disease? Am. J. Physiol. Endocrinol. Metab. 308, E335–E350 (2015).
Fick, T. A. & Backes, C. H. Cardiovascular remodeling in the small for gestational age infant - implications and future directions. Circ. J. 80, 2096–2097 (2016).
Cohen, E., Wong, F. Y., Horne, R. S. & Yiallourou, S. R. Intrauterine growth restriction: impact on cardiovascular development and function throughout infancy. Pediatr. Res. 79, 821–830 (2016).
Kaitu’u-Lino, T. J. et al. Circulating SPINT1 is a biomarker of pregnancies with poor placental function and fetal growth restriction. Nat. Commun. 11, 2411 (2020).
Dutton, P. J. et al. Predictors of poor perinatal outcome following maternal perception of reduced fetal movements–a prospective cohort study. PLoS ONE 7, e39748 (2012).
Heazell, A. E., Whitworth, M., Duley, L. & Thornton, J. G. Use of biochemical tests of placental function for improving pregnancy outcome. Cochrane Database Syst. Rev. 11, CD011202 (2015).
Goto, E. Meta-regression analysis to evaluate relationships between maternal blood levels of placentation biomarkers and low delivery weight. Int. J. Gynaecol. Obstet. 142, 148–155 (2018).
Wells, G. A. et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (2014). Accessed 11 May 2020.
Egger, M., Davey Smith, G., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634 (1997).
Akinbiyi, A. A. Unexplained elevated maternal serum alpha-fetoprotein in singleton pregnancies as a predictor of fetal risk. Int. J. Gynaecol. Obstet. 53, 17–21 (1996).
Audibert, F., Benchimol, Y., Benattar, C., Champagne, C. & Frydman, R. Prediction of preeclampsia or intrauterine growth restriction by second trimester serum screening and uterine Doppler velocimetry. Fetal Diagn. Ther. 20, 48–53 (2005).
Başbuğ, D., Başbuğ, A. & Gülerman, C. Is unexplained elevated maternal serum alpha-fetoprotein still important predictor for adverse pregnancy outcome? Ginekol. Pol. 88, 325–330 (2017).
Brazerol, W. F., Grover, S. & Donnenfeld, A. E. Unexplained elevated maternal serum alpha-fetoprotein levels and perinatal outcome in an urban clinic population. Am. J. Obstet. Gynecol. 171, 1030–1035 (1994).
Buckland, C. M., Thom, H. & Campbell, A. G. Maternal serum alpha-fetoprotein levels in low birth weight singleton pregnancies. J. Perinat. Med. 12, 127–132 (1984).
Capeless, E. L., Kelleher, P. C. & Walters, C. P. Elevated maternal serum alpha-fetoprotein levels and maternal risk factors. Their association with pregnancy complications. J. Reprod. Med. 37, 257–260 (1992).
Cho, S., Durfee, K. K., Keel, B. A. & Parks, L. H. Perinatal outcomes in a prospective matched pair study of pregnancy and unexplained elevated or low AFP screening. J. Perinat. Med. 25, 476–483 (1997).
Di, M. M. et al. A simultaneous evaluation of second trimester serum AFP, hCG and unconjugated oestriol as predictors of small for gestational age births. Ital. J. Gynaecol. Obstet. 10, 155–159 (1998).
Dugoff, L. et al. Quad screen as a predictor of adverse pregnancy outcome. Obstet. Gynecol. 106, 260–267 (2005).
Duric, K. et al. Second trimester total human chorionic gonadotropin, alpha-fetoprotein and unconjugated estriol in predicting pregnancy complications other than fetal aneuploidy. Eur. J. Obstet. Gynecol. Reprod. Biol. 110, 12–15 (2003).
Gordon, Y. B. et al. Maternal serum alpha-fetoprotein levels as an index of fetal risk. Am. J. Obstet. Gynecol. 133, 422–424 (1979).
Güdücü, N., Gönenç, G., Işçi, H., Yiğiter, A. B. & Dünder, I. Can quadruple test parameters predict SGA infants? J. Obstet. Gynaecol. 33, 269–271 (2013).
Hamilton, M. P., Abdalla, H. I. & Whitfield, C. R. Significance of raised maternal serum alpha-fetoprotein in singleton pregnancies with normally formed fetuses. Obstet. Gynecol. 65, 465–470 (1985).
Kuo, P. L., Lin, C. C., Lin, Y. H. & Guo, H. R. Placental sonolucency and pregnancy outcome in women with elevated second trimester serum alpha-fetoprotein levels. J. Formos. Med. Assoc. 102, 319–325 (2003).
Legge, M., Duff, G. B., Pike, L. & Aickin, D. R. Second trimester maternal serum alpha-fetoprotein as an indicator of fetal risk. Aust. NZ J. Obstet. Gynaecol. 25, 266–268 (1985).
Morssink, L. P., Kornman, L. H., Beekhuis, J. R., De Wolf, B. T. & Mantingh, A. Abnormal levels of maternal serum human chorionic gonadotropin and alpha-fetoprotein in the second trimester: relation to fetal weight and preterm delivery. Prenat. Diagn. 15, 1041–1046 (1995).
Pandey, J., Talib, V. H., Loganey, D. & Yadav, S. Diagnostic value of maternal serum alpha fetoprotein for predicting pregnancy outcome in high risk pregnancies: an epidemiological perspective. Indian J. Pathol. Microbiol. 36, 104–109 (1993).
Puntachai, P. et al. Associations between pregnancy outcomes and unexplained high and low maternal serum alpha-fetoprotein levels. Arch. Gynecol. Obstet. 292, 81–85 (2015).
Rodeck, C. H., Campbell, S. & Biswas, S. Maternal plasma alpha-fetoprotein in normal and complicated pregnancies. Br. J. Obstet. Gynaecol. 83, 24–32 (1976).
Roop, A. P., Boughman, J. A. & Blitzer, M. G. Study of the relationship between elevated maternal serum alpha-fetoprotein and adverse pregnancy outcome. MD Med. J. 40, 779–784 (1991).
Secher, N. J., Eriksen, P. S., Hansen, P. K., Lenstrup, C. & Thisted, J. High maternal serum alpha-fetoprotein and low birthweight. A study concerning possible correlation between maternal weight, smoking, and serum alpha-fetoprotein and subsequent birthweight and gestational age. Am. J. Perinatol. 2, 78–81 (1985).
Smith, M. L. Raised maternal serum alpha-fetoprotein levels and low birth weight babies. Br. J. Obstet. Gynaecol. 87, 1099–1102 (1980).
Weiner, C. P., Grant, S. S. & Williamson, R. A. Relationship between second trimester maternal serum alpha-fetoprotein and umbilical artery Doppler velocimetry and their association with preterm delivery. Am. J. Perinatol. 8, 263–268 (1991).
Wenstrom, K. D. et al. Prediction of pregnancy outcome with single versus serial maternal serum alpha-fetoprotein tests. Am. J. Obstet. Gynecol. 167, 1529–1533 (1992).
Wenstrom, K. D., Owen, J., Davis, R. O. & Brumfield, C. G. Prognostic significance of unexplained elevated amniotic fluid alpha-fetoprotein. Obstet. Gynecol. 87, 213–216 (1996).
Williams, M. A. et al. Elevated maternal serum alpha-fetoprotein levels and midtrimester placental abnormalities in relation to subsequent adverse pregnancy outcomes. Am. J. Obstet. Gynecol. 167, 1032–1037 (1992).
Yefet, E., Kuzmin, O., Schwartz, N., Basson, F. & Nachum, Z. Predictive Value of Second-trimester biomarkers and maternal features for adverse pregnancy outcomes. Fetal Diagn. Ther. 42, 285–293 (2017).
Schünemann, H., Brożek, J., Guyatt, G. & Oxman, A. GRADE handbook. https://gdt.gradepro.org/app/handbook/handbook.html#h.trgki08omk7z (2013). Accessed 11 May 2020.
Egger, M., Smith, G. D. & Altman, D. G. Systematic Reviews in Healthcare: Meta-analysis in Context 2nd edn (BMJ, London, 2007).
Acknowledgements
The author is grateful to the staff of the Medical Library, the Japan Medical Association (Tokyo, Japan) for help in retrieving the full texts of the articles included in the analysis. A native English language check was performed by Dolphin Corporation (1005 Kichijyoji Nagatami City Plaza 1-20-1 Kichijyojihonmachi. Musashino, 180-0004, Japan, trust@dolphin-tr.com).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing interests.
Patient consent
Patient consent was not required, because this study did not use the primary data of human subjects.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Goto, E. Association of high maternal blood alpha-fetoprotein level with risk of delivering small for gestational age: a meta-analysis. Pediatr Res 89, 1742–1750 (2021). https://doi.org/10.1038/s41390-020-01124-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-020-01124-8