Abstract
Introduction
Data regarding neonatal arterial ischemic stroke (NAIS) topography are still sparse and inaccurate. Despite the importance of locating NAIS to predict the long-term outcome of neonates, a map of arterial territories is not yet available. Our aim was therefore to generate the first three-dimensional map of arterial territories of the neonatal brain (ATNB) and test its usefulness.
Methods
Three-dimensional time-of-flight magnetic resonance angiography images were acquired from four neonates without NAIS. Arteries were semi-automatically segmented to build a symmetric arterial template. This allowed us to delineate the volumetric extension of each arterial territory, giving rise to the ATNB map, which is publicly available. Its applicability was tested on a sample of 34 neonates with NAIS.
Results
After applying the ATNB map to the neonatal sample, the posterior trunk of the middle cerebral artery, followed by its anterior trunk, were identified as the most affected arterial territories. When comparing the results obtained employing the map with the original diagnoses made during the standard clinical evaluation of NAIS, major diagnostic errors were found in 18% of cases.
Conclusion
The ATNB map has been proven useful to precisely identify the arterial territories affected by an NAIS, as well as to increase the accuracy of clinical diagnoses.
Similar content being viewed by others
Introduction
The value of knowing the topographic distribution of neonatal arterial ischemic strokes (NAIS) in the middle cerebral artery (MCA) for predicting motor prognosis is well recognized. In particular, lesions involving the corticospinal tract are associated with poor motor outcome.1,2,3,4 However, predicting other disabilities, such as cognitive impairment, alteration of executive functions, or language delay, as well as potential deficits associated with other arterial territories, still represents a major challenge.5,6 Accurately locating the affected vascular territories is crucial for the radiological assessment of arterial ischemic infarction and would allow for a more precise determination of the relationship between the location of the NAIS and future impairments.
Despite the importance of the location of NAIS in assessing its potential consequences, a neonatal map of arterial territories is not available. Such a map would allow clinicians to identify the vascular territory involved, helping to determine the artery segment or trunk of the main cerebral arteries affected, and assisting in distinguishing an arterial ischemic stroke from infarctions in watershed areas, venous infarctions, and other pathologies. Moreover, the accurate location of the injury derived from a stroke would allow us to provide a more precise and objective prognosis for each patient. In the research practice, establishing the exact location of a given lesion would help to standardize the results derived from different studies.
Considering the relatively high incidence of stroke in neonates (one per 3500 live births, approximately),7 along with the importance of precisely locating the lesion resulting from the stroke, we aimed to generate a three-dimensional map of arterial territories of the neonatal brain (ATNB), as well as to test its usefulness in a cohort of neonates with NAIS. Currently, intravenous or intraarterial digital subtraction angiography (IV/IA-DSA) is the preferred method to delimit the cerebral arterial territories, but this technique is too invasive for neonates.8,9 However, there are non-invasive methods that are useful for the quantification of cerebral blood flow that do not require the administration of intravascular contrast agents or radioactive labeled tracers.10 For this study, we employed magnetic resonance angiography (MRA), a non-invasive technique that is the standard to visualize the cerebral vascular tree in neonates11,12,13 and which is more suitable for this population.
Materials and methods
Creation of the map of arterial territories
Three-dimensional time-of-flight magnetic resonance angiography (3D-TOF-MRA) images were acquired from the first four consecutive neonates studied with 3 T magnetic resonance imaging (MRI) in a single tertiary hospital (Hospital Sant Joan de Déu) who did not present an NAIS or other conditions that could alter cerebral perfusion. All four neonates showed only minimal imaging alterations that did not affect the vascular distribution of the brain. Clinical information about these four neonates can be found in Supplementary Table I. The families of these neonates gave written permission to use the TOF sequence for this study. TOF-MRA imaging was performed on a Philips Ingenia 3 T scanner at Hospital Sant Joan de Déu using a neonatal head coil. The TOF-MR acquisition protocol was the following: repetition time (TR) = 23 ms, echo time (TE) = 3.45 ms, voxel size = 0.3 × 0.3 × 0.6 mm3, field of view (FOV) = 15 cm, flip angle = 18°. Arterial segmentation from the TOF images was performed semi-automatically using the thresholding method of the ITK-SNAP software, version 3.0 (http://itksnap.org).14 Once the segmentation was attained, the “extract objects” function of the software MRIcroGL (http://mccauslandcenter.sc.edu/mricrogl) with an Otsu level of 3 and edge dilation of 2 was employed to extract the single largest object and reduce the potential noise from the segmentation image. Afterwards, the TOF image of each neonate was registered to the corresponding 3D image of the participant by means of the flirt function of the FSL suite, version 5.0 (http://fsl.fmrib.ox.ac.uk). The 3D image of each participant was then elastically registered to a standardized neonatal brain15 using the ANTS software (http://picsl.upenn.edu/software/ants).16 The parameters of these registrations were then applied to the arterial segmentation image of each participant, which eventually allowed us to have all the arterial segmentations in a common space. The four arterial segmentation images were then flipped (i.e., left–right hemispheres were reversed), and the original and flipped segmentation images were used to build a symmetric arterial template with the ANTS software. This template allowed us to delineate the volumetric extension of each arterial territory, which was done by hand by three of the researchers (G.A., C.S.-O. and A.G.-A.) using ITK-SNAP, which gave rise to the map of ATNB.
Patients
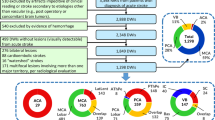
The ATNB map was tested on a cohort of 34 patients with NAIS (12 females), consecutively admitted to a single tertiary neonatal unit (Hospital Sant Joan de Déu - Hospital Clinic, Barcelona) between 2010 and 2016. The inclusion criteria were newborn infants (>36 gestational weeks) with symptomatic NAIS and without underlying pathology, such as sepsis, meningitis, congenital heart disease, and so on. Symptomatic NAIS was defined as the finding of an ischemic lesion, confirmed by MRI, in the territory of the main cerebral arteries [anterior cerebral artery (ACA), MCA, and/or posterior cerebral artery (PCA)], in an infant presenting seizures, recurrent apnea, or acute neurological dysfunction before 28 days after birth. This sample was previously assessed in a recent study by our group.17 The medical ethics committee of our institution approved the study and parents of all the subjects signed an informed consent. The original sample consisted of 43 neonates, but 9 were excluded for different reasons (2 did not have the informed consent, 4 had a congenital heart disease, and 3 had genetic disorders: 2 Down syndrome and 1 incontinentia pigmenti).
MRI data acquisition
MRI was performed on a General Electric 1.5 T Signa Excite scanner at Hospital Sant Joan de Déu using a neonatal head coil, following the clinical MRI protocol for neonates with suspected NAIS. Median age at MRI acquisition was 5 days [interquartile region (IQR) = 5]. The MRI acquisition protocol was the following; structural T1: TR = 12.64 ms, TE = 5.75 ms, voxel size = 0.4 × 0.4 × 1.0 mm3, FOV = 20 cm; diffusion-weighted images: TR = 8300 ms, TE = 94.1 ms, voxel size = 0.8 × 0.8 × 5.0 mm3, FOV = 20 cm, b = 1000; axial T2: TR = 3000 ms, TE = 11 ms, voxel size = 0.31 × 0.31 × 6.0 mm3, FOV = 16 cm; coronal T2: TR = 4560 ms, TE = 65 ms, voxel size = 0.31 × 0.31 × 4.0 mm3, FOV = 16 cm. The flip angle for T1 was 20°, and 90° for the rest.
Individual MRI image analysis
The lesion segmentation process was performed by two of the researchers (C.N. and C.S.-O.) through multimodal analysis of MRI images with ITK-SNAP. In order to simultaneously display images from the different acquisition modalities, the linear transformation flirt function of the FSL suite was used; for each subject, the diffusion, coronal T2, and axial T2 images were registered to the more anatomically precise T1 image. Then, the region competition preprocessing function of ITK-SNAP was applied using five-tissue clustering, 0.5 region competition force, and 0.5 smoothing force. Once a satisfactory lesion segmentation was attained in the “evolution” step, it was manually corrected by simultaneously inspecting its features in all the imaging modalities, as well as its 3D visualization. Finally, each lesion segmentation was elastically registered to the ATNB map and quantified.
Review of clinical diagnoses
The arterial territory classification made in the clinical setting during the admission of the neonates with NAIS was compared with the results obtained employing the ATNB map. Discrepancies between the two classifications were thoroughly reviewed by three investigators of the research team (G.A., T.A. and A.G.-A.), who were blind to both the clinical classification and the classification provided by the ATNB map. The final classification reached by consensus among these three researchers for each case was considered the reference. This was intended to be an indicator of how many of the clinical diagnoses were imprecise, incomplete, or erroneous, and to what extent the ATNB map could help to improve or correct such diagnoses. Two types of error were considered: major errors (misclassifications, lack of important information), and minor errors (inaccuracies, lack of detail). Examples of both types of error can be found in Supplementary Table II.
Automated classification tool
Along with the ATNB map, a computer application based on this map is provided, which allows automatically locating one or more previously segmented lesions and quantifying volume involvement in the associated territory. This tool is publicly available for download in the nitrc.org website (https://www.nitrc.org/projects/atnb_classif), along with the ATNB map in NIfTI format. Detailed information about this application and how to use it is available in Supplementary Methods I.
Results
A preview of the ATNB map is shown in Fig. 1. The complete and high-resolution version of this map, available for printing purposes, may be downloaded from the nitrc.org website (https://www.nitrc.org/projects/atnb_classif) and is presented in Supplementary Fig. I. The resulting arterial segmentation that allowed the creation of the ATNB map is presented in Fig. 2.
ACA: anterior cerebral artery; Perf. ACA: perforating branches of the anterior cerebral artery; Ant. MCA: anterior branch of the middle cerebral artery; Post. MCA: posterior branch of the middle cerebral artery; Perf. MCA: perforating branches of the middle cerebral artery; PCA: posterior cerebral artery; Perf. PCA: perforating branches of the posterior cerebral artery; CA: choroidal artery; SCA: superior cerebellar artery; AICA: anterior inferior cerebellar artery; PICA: posterior inferior cerebellar artery.
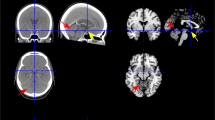
In order to analyze the applicability of the ATNB map in clinical practice, it was used to classify lesions in our cohort of 34 neonates with NAIS. An example of how a particular NAIS fits in the map is depicted in Fig. 3. Our results showed that the posterior trunk of the MCA (pMCA), followed by the anterior trunk of the MCA (aMCA), were the arterial territories in which the most patients had lesions (29 and 20, respectively) (Fig. 4c).Footnote 1 Accordingly, these two regions were also the most broadly affected; considering the volume of all the lesions added up, 57% of the total lesion volume involved the pMCA territory, whereas 24% involved the aMCA territory (Fig. 4a, b). Notably, 16 neonates (47%) had lesions involving more than one arterial territory. Although the left hemisphere was more frequently affected than the right (18 lesions were located in the left hemisphere, 11 in the right hemisphere, and 5 were bilateral), the pattern of territory involvement was highly symmetrical between hemispheres (Fig. 4b).
a Pie chart depicting which arterial territories are most frequently involved in NAIS, considering the sum of all lesion volumes. b Graphical representation of which arterial territories are most frequently involved (dark blue), for each hemisphere. Only the four most frequently affected arterial territories are depicted. c Bar chart depicting number of cases in which a given arterial territory is involved. A particular territory was considered to be affected by the lesion if the following criteria were met: at least an extension of 15% of the territory was involved or at least 15% of the lesion involved the territory. Note that the number of arterial territories affected is greater than the number of patients because some neonates had multiple territories involved. Please refer to Fig. 1 caption for abbreviations.
To assess the potential impact of the application of the map in a clinical setting, we quantified the degree of agreement with the diagnostic team during standard clinical evaluation. Discrepancies between the classifications provided by the ATNB map and those made in the clinical setting were reviewed by three researchers. The classification reached by consensus among the research team was always congruent with the classification provided by the ATNB map. It was therefore concluded that only 13 out of the 34 (38%) NAIS analyzed were perfectly identified by the diagnostic team, while the rest of the assessments (62%) were considered either imprecise or incomplete, that is, with minor errors (15/34, 44%); or were misclassified or lacked essential information, that is, with major errors (6/34, 18%). A case-by-case list of the diagnoses made by the diagnostic team and their accuracy when compared with the results derived from the application of the ATNB map and reviewed by the three researchers is presented in Supplementary Table III. Consider, by way of example, the case documented in Supplementary Table IIA, in which the lesion was erroneously identified during the clinical evaluation as being located at the PCA, while it was actually located at the ACA.
Discussion
To our knowledge, this is the first map of arterial territories specifically created for the neonatal brain. The methodology employed to create this map, which entailed the creation of a template based on the arterial segmentation of four neonates without NAIS, ensures that it reliably represents the normal vascular distribution of the neonatal brain. This map facilitates identification of the arterial territories affected by an NAIS. Given the importance of stroke location when predicting the long-term neurological outcome of the neonate,3,18,19 we expect this map to be useful for clinicians and radiologists. It has the potential to limit the number of inaccuracies resulting from the use of two-dimensional images, thereby minimizing the number of errors in diagnosis and therefore in formulating the prognosis. We expect the map to be helpful in research practice too, as it allows for the creation of a common and standardized workspace that will facilitate interpreting the results of future investigations.
According to the ATNB map, 88% and 59% of the NAIS of our sample involved the posterior and anterior branches, respectively, of the MCA. This territory was consequently the most affected, which is consistent with previous reports,3,20 although other territories were also involved in a lower proportion of cases and to a lesser extent. In fact, nearly half of the neonatal sample (47%) had lesions in more than one arterial territory. It is important to note, however, that only 21% of all the infarcts were multifocal. In other words, in most of those cases in which more than one arterial territory was involved, a single lesion affected contiguous territories. In any case, lesions involving territories other than the one of the MCA could provide additional useful information when determining the outcome of newborns and must be considered as well. In this regard, the ATNB map allowed us to ascertain that the territory of the PCA was, strikingly, greatly affected, as nearly a third of the newborns presented lesions there. It is important to keep in mind, however, that cerebral plasticity mechanisms may overcome the effects of a lesion, as has already been shown in the case of language functions.21 Moreover, the prediction of non-motor impairments is still challenging.5,6 Therefore, caution should be taken when making an outcome prediction, even when having precise topographic information of the NAIS.
Even though there is no gold standard against which to compare the classifications provided by the ATNB map, it is encouraging to learn that these classifications were always congruent with those carefully provided by the research team, which were considered the reference. In this regard, the ATNB map was crucial in revealing and amending some errors in diagnosis made by experienced medical staff in the clinical setting. In particular, 6 out of the 34 (18%) NAIS studied were misclassified or lacked some important information, while 15 out of the 34 (44%) cases were seen to have at least one minor error in the diagnosis, such as incomplete information or slight inaccuracies. It is remarkable that the original diagnosis made by the clinical staff missed 10 of the 11 lesions (91%) shown by the ATNB map to also affect the PCA. This further highlights the importance of the 3D ATNB map presented here as a tool to help radiologists and neonatologists avoid NAIS misclassification and/or accurately locate lesions, thereby ensuring a more precise prognosis. The ATNB map is particularly helpful when analyzing the extension of the lesions over the arterial territories, which is often difficult to determine. Overall, these results lend support to the potential use of the map of arterial territories in both research and clinical practice.
Here we also present an automated protocol that employs the ATNB map to classify each NAIS in the corresponding arterial territories by providing a 3D image of the neonatal brain and the segmented lesion. This application may be useful for researchers, as well as for clinicians and radiologists, as it speeds up the classification process while procuring an unbiased approach. Moreover, it may be considered as the final step of a potentially fully automated procedure aiming to recognize and classify neonatal strokes as soon as MRI data have been acquired. The first step in this procedure would involve automatic segmentation of the stroke, toward which end there are projects currently underway.22 Ideally, in the near future these two steps may be joined together in a single application to allow for a fast, accurate, and unbiased diagnostic procedure.
The present study has some limitations that must be pointed out. First, the smaller brain vessels of the neonates are often difficult to visualize with MRA. Despite this, we employed a 3 T scanner that enables to identify the vascular tree of the brain more accurately than other scanners with smaller magnetic field strength, which in turn allowed us to delineate the volumetric extension of the arterial territories quite precisely. Second, we did not take into account the potential variability of the anatomical distribution of the arterial territories from one neonate to another.
Future versions of this map could include different templates to account for the most common variants of vascular distribution, as well as the representation of essential anatomical structures frequently affected by neonatal strokes. Moreover, the precise location of the NAIS with the ATNB map, along with recent imaging technologies measuring white matter integrity and connectivity, may provide additional information on the development of cerebral functions after the stroke. Since this is the first map of arterial territories of the neonatal brain, it should be tested with larger samples in order to further assess its accuracy and perhaps slightly redefine its boundaries, if necessary, in a later version. Additional research on different age groups may also be of interest in order to explore how the arterial territories evolve throughout life.
Summary
This is the first 3D map of arterial territories of the neonatal brain. It has been proven to be useful in research and may be of clinical value as well, since it facilitates identification of the arterial territories affected by a neonatal stroke, which is of great importance when predicting the neurological outcome of the newborn. This map could be useful to distinguish an ischemic arterial stroke from infarctions in watershed areas and venous infarctions.
Notes
To perform this calculation, a particular territory was considered to be affected by the lesion if the following criteria were met: at least an extension of 15% of the territory was involved or at least 15% of the total lesion volume involved the territory.
References
De Vries, L. S., Van der Grond, J., Van Haastert, I. C. & Groenendaal, F. Prediction of outcome in new-born infants with arterial ischaemic stroke using diffusion-weighted magnetic resonance imaging. Neuropediatrics 36, 12–20 (2005).
Domi, T. et al. Corticospinal tract pre-wallerian degeneration: a novel outcome predictor for pediatric stroke on acute MRI. Stroke 40, 780–787 (2009).
Husson, B. et al. Motor outcomes after neonatal arterial ischemic stroke related to early MRI data in a prospective study. Pediatrics 126, 912–918 (2010).
Dinomais, M. et al. Long term motor function after neonatal stroke: lesion localization above all. Hum. Brain Mapp. 36, 4793–4807 (2015).
deVeber, G. A. et al. Epidemiology and outcomes of arterial ischemic stroke in children: The Canadian Pediatric Ischemic Stroke Registry. Pediatr. Neurol. 69, 58–70 (2017).
Wagenaar, N. et al. Neurodevelopment after perinatal arterial ischemic stroke. Pediatrics 142, e20174164 (2018).
Agrawal, N., Johnston, S. C., Wu, Y. W., Sidney, S. & Fullerton, H. J. Imaging data reveal a higher pediatric stroke incidence than prior US estimates. Stroke 40, 3415–3421 (2009).
Burger, I. M., Murphy, K. J., Jordan, L. C., Tamargo, R. J. & Gailloud, P. Safety of cerebral digital subtraction angiography in children: complication rate analysis in 241 consecutive diagnostic angiograms. Stroke 37, 2535–2539 (2006).
Buhk, J. H., Lingor, P. & Knauth, M. Angiographic CT with intravenous administration of contrast medium is a noninvasive option for follow-up after intracranial stenting. Neuroradiology 50, 349–354 (2008).
Goff, D. A., Buckley, E. M., Durduran, T., Wang, J. & Licht, D. J. Noninvasive cerebral perfusion imaging in high-risk neonates. Semin. Perinatol. 34, 46–56 (2010).
Rodallec, M. H., Marteau, V., Gerber, S., Desmottes, L. & Zins, M. Craniocervical arterial dissection: spectrum of imaging findings and differential diagnosis. Radiographics 28, 1711–1728 (2008).
Siddiq, I. et al. Utility of neurovascular imaging in acute neonatal arterial ischemic stroke. J. Pediatr. 188, 110–114 (2017).
Arnaez, J. & Garcia-Alix, A. Extracerebral thrombosis in symptomatic neonatal arterial ischemic stroke. Eur. J. Paediatr. Neurol. 21, 687–688 (2017).
Yushkevich, P. A. et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage 31, 1116–1128 (2006).
Sanchez, C. E., Richards, J. E. & Almli, C. R. Age-specific MRI templates for pediatric neuroimaging. Dev. Neuropsychol. 37, 379–399 (2012).
Avants, B. B. et al. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage 54, 2033–2044 (2011).
Stephan-Otto, C., Núñez, C., Arca, G., Agut, T. & García-Alix, A. Three-dimensional map of neonatal arterial ischemic stroke distribution from early multimodal brain imaging. Stroke 48, 482–485 (2017).
Kirton, A., Deveber, G., Pontigon, A. M., Macgregor, D. & Shroff, M. Presumed perinatal ischemic stroke: vascular classification predicts outcomes. Ann. Neurol. 63, 436–443 (2008).
François, C. et al. Language learning and brain reorganization in a 3.5-year-old child with left perinatal stroke revealed using structural and functional connectivity. Cortex 77, 95–118 (2016).
Kirton, A., Shroff, M., Visvanathan, T. & deVeber, G. Quantified corticospinal tract diffusion restriction predicts neonatal stroke outcome. Stroke 38, 974–980 (2007).
François, C. et al. Right structural and functional reorganization in four-year-old children with perinatal arterial ischemic stroke predict language production. eNeuro 6, ENEURO.0447–18.2019 (2019).
Pustina, D. et al. Automated segmentation of chronic stroke lesions using LINDA: lesion identification with neighborhood data analysis. Hum. Brain Mapp. 37, 1405–1421 (2016).
Acknowledgements
This work was supported by Grants PI08/1366 and PI15/00846 from the Instituto de Salud Carlos III co-funded by the European Regional Development Fund.
Author information
Authors and Affiliations
Contributions
C.N. wrote the manuscript. C.N. and C.S.-O. created the map and analyzed the sample of neonates with NAIS. G.A., T.A. and A.G.-A. collected the data and analyzed MR images. C.S.-O. and A.G.-A. designed the study. All authors participated in the interpretation of the results. All authors reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Núñez, C., Arca, G., Agut, T. et al. Precise neonatal arterial ischemic stroke classification with a three-dimensional map of the arterial territories of the neonatal brain. Pediatr Res 87, 1231–1236 (2020). https://doi.org/10.1038/s41390-019-0724-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-019-0724-x