Abstract
Background
There is an association between hypocapnia and adverse neurodevelopmental outcome in infants with neonatal encephalopathy (NE). Our aim was to test the safety and feasibility of 5% CO2 and 95% air inhalation to correct hypocapnia in mechanically ventilated infants with NE undergoing therapeutic hypothermia.
Methods
Ten infants were assigned to this open-label, single-center trial. The gas mixture of 5% CO2 and 95% air was administered through patient circuits if the temperature-corrected PCO2 ≤40 mm Hg. The CO2 inhalation was continued for 12 h or was stopped earlier if the base deficit (BD) level decreased <5 mmol/L. Follow-up was performed using Bayley Scales of Infant Development II.
Results
The patients spent a median 95.1% (range 44.6–98.5%) of time in the desired PCO2 range (40–60 mm Hg) during the inhalation. All PCO2 values were >40 mm Hg, the lower value of the target range. Regression modeling revealed that BD and lactate had a tendency to decrease during the intervention (by 0.61 and 0.55 mmol/L/h, respectively), whereas pH remained stable. The rate of moderate disabilities and normal outcome was 50%.
Conclusions
Our results suggest that inhaled 5% CO2 administration is a feasible and safe intervention for correcting hypocapnia.
Similar content being viewed by others
Introduction
Neonatal encephalopathy (NE) continues to be one of the leading causes of neonatal mortality and morbidity worldwide.1 Although therapeutic hypothermia (33.5 °C) (HT) has been clearly proven to reduce mortality and adverse neurodevelopmental outcome in patients with moderate-to-severe NE,2,3 there is a need for further interventions in order to optimize neuroprotection.
Multiple analyses reported a high rate of hypocapnia during the early hours of postnatal life among asphyxiated infants.4,5,6,7,8 Furthermore, a growing body of evidence describes an association between hypocapnia and adverse neurodevelopmental outcome. First, preclinical studies have established that hypocapnia negatively affected the cellular energy metabolism of the brain, resulting in increased apoptosis.9,10 Also, hypocapnia during the first postnatal days is associated with periventricular leukomalacia and cerebral palsy in preterm neonates.11,12 The potential harmful effects of hypocapnia are further demonstrated by retrospective analyses of resuscitated adults following cardiac arrest, reporting that normocapnia or mild hypercapnia was associated with better neurological outcomes compared to hypocapnia.13,14 Most importantly, the secondary analysis of two large HT trials showed a dose-dependent association between hypocapnia and the combined outcome of death and neurodevelopmental disability in a mixed population of cooled and non-cooled NE infants after adjusting for the indicators of disease severity.4,5 The consistent findings on the independent association between neurological impairment and low levels of partial pressure of carbon dioxide (PCO2) suggest that it may be advisable to avoid hypocapnia in infants with NE.
However, clinicians have limited options to lower the risk of hypocapnia in asphyxiated infants, who tend to spontaneously hyperventilate due to severe metabolic acidosis.15,16 Beside muscle relaxation, which has well-known side effects,17 inhalation of low concentration carbon dioxide (CO2) could be a reasonable approach to avoid hypocapnia in this patient population. Inhaled CO2 has been tested in several indications in pediatric and neonatal patients.18,19,20,21,22,23
The aim of this study was to evaluate the safety and feasibility of adding CO2 to the inhaled gas mixture in low concentration (5% CO2 + 95% air) to achieve a desired range of PCO2 of 40–60 mm Hg in mechanically ventilated, hypocapnic infants undergoing HT for NE.
Methods
Subjects
This was an open-label, single-center trial, conducted at the 1st Department of Paediatrics, Semmelweis University, Budapest, Hungary, a tertiary neonatal center treating out-born patients only. We recruited 10 infants from February 2016 to June 2017.
The study protocol was approved by Scientific and Medical Research Council Ethics Committee of Hungary (5705-1/2016/EKU). The study was registered with ClinicalTrials.gov number NCT02700854 entitled Hypoxic-Ischemic Encephalopathy Therapy Optimization in Neonates for Better Neuroprotection with Inhalative CO2 (HENRIC). An external Data and Safety Monitoring Committee consisting of four independent neonatologists reviewed the data after each patient enrolment and permitted the continuation of the study. Infants with moderate and severe encephalopathy fulfilling the criteria of HT treatment according to parameters set by the Total Body Hypothermia for Neonatal Encephalopathy Trial protocol24 were eligible for enrolment. The local protocol was to mechanically ventilate all infants undergoing therapeutic HT. Inclusion criteria were: (1) temperature-corrected arterial PCO2 ≤ 40 mm Hg at any time within 6 h of life; (2) presence of spontaneous respiratory efforts while being intubated and ventilated; and (3) presence of an indwelling arterial line.
Exclusion criteria were (1) meconium aspiration syndrome or an oxygen requirement > 40%; (2) severe metabolic acidosis (pH < 6.8 and/or lactate levels > 15 mM) on admission; (3) cardiovascular compromise requiring more than one inotropic agent; (4) anemia (hematocrit < 35%); and (5) >1 mmol/kg bicarbonate administration during initial stabilization; and (6) major birth defects.
Written informed consent was obtained from a parent of each infant after explanation of the study. According to the study protocol, CO2 inhalation was discontinued after 12 h or earlier, if the base deficit (BD) decreased < 5 mmol/L. The targeted PCO2 range was between 40 and 60 mm Hg.
Protocol
The gas mixture of 5% CO2 and 95% air (N-carbogen, Messer Hungarogaz Kft, Budapest, Hungary) was administered into the inspiratory arm of the patient’s ventilator circuit (Fabian, Acutronic Medical System, Hirzel, Switzerland). The fraction of inspired oxygen could be titrated to maintain peripheral oxygen saturation (SpO2) between 90% and 96%. Initial parameters of mechanical ventilation were set according to the local protocol: synchronized intermittent mandatory ventilation with volume guarantee (target tidal volume 5 ml/kg, respiratory rate (RR) 20/min, positive end expiratory pressure 5 cm H2O, peak inflating pressure (PIP) limit (Pmax) was set 5 cm H2O above the “working” PIP, inspiratory time 0.35–0.4 s, with an inspiratory and expiratory circuit flow of 7–8 L/min). The inspiratory and expiratory flow values were not changed during the study period. To monitor CO2 delivery, a CO2 sampling line was built in the inhalation circuit, which was connected to an external end-tidal CO2 module (Covidien Microstream, MicroPod, Acutronic Medical System, Hirzel, Switzerland) of the Fabian ventilator. The ventilator displayed the partial pressure of the inhaled 5% CO2, which was equal to 36 mm Hg at atmospheric pressure.
During the CO2 exposure, arterial blood gas samples were taken initially every 30 min, while after the stabilization of arterial PCO2, sampling was continued 2-hourly until the end of the inhalation. Carbon dioxide tension, partial pressure of oxygen (PO2), and pH were all temperature corrected.25 Ventilation settings were only changed if PCO2 fell < 35 or increased > 65 mm Hg. The protocol also instructed the PCO2 administration to be stopped if the PCO2 increased > 85 mm Hg.
Cardiorespiratory parameters and amplitude-integrated electroencephalogram (aEEG) background and seizure activity were monitored closely during the study. Transcranial Doppler ultrasound measurements of cerebral blood flow velocity (CBFV) in the anterior and middle cerebral arteries (ACA and MCA, respectively) were performed before, during, and after the CO2 exposure. Brain magnetic resonance imaging (MRI) studies were carried out within the first week of life.
Outcomes
The primary outcome was the percentage of time spent in the desired temperature-corrected PCO2 range of 40–60 mm Hg during CO2 inhalation. The secondary outcomes were defined as: (1) hours of life until BD decreased < 5 mmol/L; (2) hours of life until pH increased > 7.25; (3) severe hypotension (mean arterial pressure < 25 mm Hg), despite administration of more than one inotropic agent and volume replacement within the 72 h of life; (4) number of seizures, either detected clinically or by aEEG monitoring within the 72 h of life; and (5) intracranial hemorrhage detected by MRI within the first week of life.
Bayley Scales of Infant Development II examination was performed at 18–22 months of age by trained examiners. Moderate disability was defined as mental developmental index (MDI) and/or psychomotor developmental index (PDI) score 1–2 standard deviation (SD) below the mean (70–84). Severe disability was defined as any of the following: severe cerebral palsy, hearing impairment, bilateral cortical visual impairment, MDI and/or PDI > 2 SD below the mean (<70).
Data analysis
To analyze the cardiorespiratory parameters and CBFV values, for each infant we calculated the mean values for continuous variables during the 3 time epochs of the study: before, during, and after the inhalation of 5% CO2 and compared the means between the 3 epochs by non-parametric repeated measurements analysis of variance (Friedman test); data are presented as medians with ranges. For categorical variables, differences were assessed using Chi-squared test. Linear interpolation was used to estimate the time spent in the desired PCO2 range throughout the CO2 inhalation. Regression modeling was performed to predict the changes of BD, pH, and lactate over time during the CO2 inhalation, using a repeated-measures linear mixed-effect model with first-order autoregressive within-group correlation structure fitted by maximizing the restricted log-likelihood. The following variables were considered to have fixed effects: blood gas value at the beginning of inhalation as baseline, time in hours since the beginning of inhalation, and Thompson encephalopathy score predicting adverse neurological outcome (low as 0, medium as 1, high as 2).26
Intra-reader reproducibility of the systolic (Vs) and diastolic peak flow velocity (Vd) measurement in ACA was evaluated on seven patients’ images before the study commencement by calculating the intra-class correlation coefficient (ICC).
Matched control patients were selected from our cooling database. Patients were matched for NE severity according to the Thompson encephalopathy score, PCO2 ≤40 mm Hg any time within the first 6 h of postnatal life, and for birth weight (<3000 g; ≥3000 g and <4000 g; ≥4000 g). We have analyzed the blood gas values from the first hypocapnic value (≤40 mm Hg) measured up to 16 h in the control group similarly to the intervention group. Similar regression modeling was employed using all patients to compare the changes of BD, pH, and lactate over time between the intervention and control groups. A “treatment” term (1 = CO2 inhalation, 0 = none) was used to assess the significance of the intervention. Baseline values, treatment, time elapse in hours, and treatment–time interaction were considered to have fixed effects.
We used the R Statistical Software 3.4.4 with significance set at p < 0.05 to analyze the data. Data were plotted by GraphPad Prism version 5.0 (GraphPad Software, La Jolla, CA).
Results
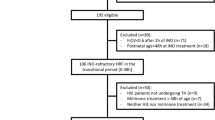
Sixty-two term infants with moderate or severe NE were assessed for eligibility, and a total of ten patients were enrolled prospectively into the trial (Supplementary Fig. S1).
Baseline clinical characteristics of the ten infants are summarized in Table 1. The median PCO2 was 33 mm Hg (range 26–40 mm Hg) at the start of 5% CO2 administration; the latter commenced at a median 5.5 h of life (range 3.9–6.6). The CO2 inhalation was stopped at a median 12.9 h of life (range 5.4–18.6), after a median duration of 7.7 h (range 0.6–12.0).
During the CO2 administration, a total of 50 arterial blood gases were taken from the 10 patients. The temperature-corrected arterial PCO2 was between the targeted 40 and 60 mm Hg in 96% (48/50) of the samples. All PCO2 values were >40 mm Hg, and the highest PCO2 value detected was 64 mm Hg (infant no. 6). Calculating with a linear interpolation between the blood gas measurements, patients spent a median 95.1% (range 44.6–98.5%) of time in the desired PCO2 range during the 5% CO2 inhalation (Table 1 and Fig. 1a). In comparison, the patients in the control group spent significantly less time (median 45.3% (range 0–91.7%), p = 0.002) in the target range (Supplementary Fig. S2).
Each symbol represents one patient. Baseline values (0-point) correspond to the last measured value prior to the start of CO2 inhalation. The last data point on the graphs correspond to values measured after the offset of CO2 administration. The x-axis displays the time in hours since the start of CO2 inhalation. a PCO2 trends for each patient during the study. Patients spent 95.1% of time (range: 44.6–98.5%) in the desired PCO2 range (40–60 mm Hg) during the 5% CO2 administration, calculated by linear interpolation between the blood gas measurements. All PCO2 values were >40 mm Hg, the lower value of the target range. b pH trends for each patient during the study. A repeated-measures linear mixed-effect model predicted that pH remained stable over time during the CO2 administration. Baseline value, time in hours since the beginning of inhalation, and Thompson encephalopathy score were considered to have fixed effects. c Base deficit trends for each patient during the study. The same model predicted that base deficit decreased by 0.61 mmol/L per hour throughout the CO2 inhalation period. d Lactate trends for each patient during the study. The same model predicted that lactate levels decreased by 0.55 mmol/L per hour throughout the CO2 inhalation period.
The 5% CO2 exposure was continued for the predefined maximum of 12 h in 4 cases, while in 6 cases, the BD decreased <5 mmol/L earlier (Table 1) and CO2 administration was stopped accordingly. It is noteworthy that the latter 6 infants’ BD normalized within 13 h of life, while in the former 4 cases the BD recovery was longer and normalized between 23.0 and 59.8 h of life (Table 1). The end point of acidosis (defined as pH > 7.25) was median 9.1 h of life with a wide range of 1.5–59.8 h. In 3/10 cases, the pH was >7.25 initially, before the inhalation was initiated and pH did not decrease below this threshold during the exposition (Fig. 1b).
The statistical modeling for trends of BD, pH, and lactate during the study period (Fig. 1a–d) revealed that baseline value at the beginning of inhalation had a significant effect on the changes of blood gas values over time. The regression equation predicted that BD decreased by 0.61 mmol/L and lactate decreased by 0.55 mmol/L per hour after the beginning of inhalation, whereas pH remained stable over time. Interestingly, the Thompson encephalopathy score, measured on a three-step scale of low, medium, and high showed significant association only with BD trends (Table 2). The matched controls showed a similar tendency in recovery from acidosis, except for the changes in pH. The pH showed a slower recovery in the intervention group by a 0.01/h (Supplementary Table S2).
Cardiovascular and respiratory parameters of the study population were analyzed at three time epochs: pre-inhalation, during, and post-inhalation (Fig. 2). We detected a statistically significant decrease in heart rate (p = 0.007) during the study, which is a physiological response to reduced body temperature. Mean arterial blood pressure and peripheral oxygen saturation did not change (Fig. 2a). Severe hypotension or cardiovascular collapse did not occur. The maximum dose of dopamine was 10 μg/kg/min within the first day of life in our study population and a median 13.5 ml/kg volume was administrated during the CO2 exposition as fluid boluses, including blood products for the correction of anemia or coagulopathy. Regarding respiratory parameters, the RR reduced significantly in the post-inhalation epoch of the study compared to the RR before and during CO2 administration (p = 0.008, Friedman test). Furthermore, we detected an increased tidal volume (p = 0.002, Skillings–Mack test) during the 5% CO2 inhalation, reflecting a physiological response to the intervention (Fig. 2b). The temperature-corrected arterial PO2 was >100 mm Hg in 40% (20/50) of the samples in 7 patients during the 5% CO2 exposition while the oxygen requirement was 21% in all cases. The highest PO2 was 141 mm Hg. Importantly, we did not observe any case of pulmonary or circulatory failure during the study period.
Data are presented as medians with ranges in the entire cohort. a Heart rate (HR) decreased from a median 108/min (87–134) to 97/min (82–122) during CO2 inhalation. After the CO2 administration, the HR reduced further to 88/min (75–120); (p = 0.007, Friedman test). The mean arterial blood pressure (MABP) and peripheral oxygen saturation (SpO2) did not change and remained in the physiological range throughout the three time epochs. (MABP medians: pre-study: 47 mm Hg (44–56); during: 47 mm Hg (40–52); post-study: 46 mm Hg (40–49); (p = 0.07, Friedman test). SpO2 medians: pre-study: 100% (98–100); during: 99% (97–100); post-study: 97% (93–100); (p = 0.07; Friedman test). b The respiratory rate and tidal volumes changed significantly over the three study epochs. Respiratory rate was 42/min (24–53) and 42/min (25–58) before and during the CO2 inhalation, respectively, and reduced to 28/min (19–49) in the post-study period (p = 0.008; Friedman test). Peak tidal volumes were the following: pre-study: 5.0 mL/kg (2.8–7.3); during: 10.3 mL/kg (5.3–16.6); and post-study: 4.9 mL/kg (3.9–8.7); (p = 0.002; Skillings–Mack test).
Continuous aEEG monitoring detected electrophysiological seizures in 3 patients during the 5% CO2 exposition, 2 of them already had seizure activity before, and all 3 had seizures after the inhalation, resulting in permanent anticonvulsive treatment as per decision of the clinical team (Table 3).
Brain MRI scans were carried out at a median of 3.5 (range 2–8) days of life. Diffusion-weighted imaging showed the presence of hypoxic–ischemic injury in 6/10 patients. Deep gray matter and white matter involvement, including corpus callosum, was noted as the most frequent type of brain injury. Six neonates were noted to have intracranial hemorrhage in subdural, subarachnoid, and intraventricular (grade I) location, but none of them developed intraparenchymal bleeding (Table 3).
Transcranial Doppler ultrasonography measurements of CBFV were performed by the same physician. Intra-reader reliability revealed excellent reproducibility for Vs (ICC = 0.899, 95% confidence interval (CI): 0.64–0.98) and Vd (ICC = 0.860, 95% CI 0.44–0.97). CBFV was measured in the ACA and MCA before the study commencement (at median 5.6 h of life (range 3.6–6.3)), every 2 h during CO2 exposition, and shortly after the cessation of CO2 (at median 17.9 h of life (range 8.2–19.2)). We present the values measured in the ACA of 7 patients (infant nos. 4–10) who were investigated at each time epoch (Fig. 3). We could not find differences in CBFV values when comparing the three epochs of the study. The MCA blood flow velocities showed similar tendencies (data are not shown).
Median values with ranges are shown for systolic peak flow velocity (Vs), end diastolic peak flow velocity (Vd), resistance index (RI), and pulsatility index (PI) of the anterior cerebral artery in the three epochs of the study. RI and PI were calculated using the following formulas: RI = (Vs − Vd)/Vs and PI = (Vs − Vd)/mean velocity. We could not find differences in CBFV values when comparing the three epochs of the study. See the text for details.
No death occurred during the perinatal period in our study population. One infant (no. 6) with severe NE subsequently died in another hospital due to aspiration pneumonia. Severe disability (PDI and/or MDI <70) occurred in three cases; two infants were classified as moderate (PDI and/or MDI 70–84) on Bayley II scales at 18–22 months of age. One child (no. 7) had a total score of 88 (=6 percentile) on the behavior rating scale indicating a non-optimal test behavior. Three infants had normal outcome (both PDI and MDI ≥85). The rate of severe and moderate disabilities did not differ between the treated and control groups (Supplementary Table S1).
Discussion
Based on our small, single-center, safety, and feasibility trial, 5% CO2 and 95% air inhalation corrected hypocapnia in asphyxiated, cooled infants with spontaneous hyperventilation as the temperature-corrected PCO2 was within the target range (40–60 mm Hg) in 95.1% of time throughout the inhalation period, and no PCO2 values were <40 mm Hg. The regression modeling predicted that BD and lactate had a tendency to decrease, whereas pH remained stable during the CO2 exposition. Matched, control patients with NE spent significantly less time in the target PCO2 range and showed a similar tendency in recovery from acidosis. We consider the minimally slower pH recovery (0.01/h) clinically insignificant. Importantly, serious adverse events were not registered during the study period and the cardiovascular and respiratory status of the neonates remained stable.
There is physiological plausibility for hypocapnia to exacerbate brain injury: low PCO2 decreases global and regional oxygen supply due to the leftward shift of oxyhemoglobin curve and also causes systemic/cerebral vasoconstriction.15 In addition, hypocapnia initiates nuclear DNA fragmentation, membrane lipid peroxidation, and apoptotic cell death in the cerebral cortex and facilitates the release of excitatory amino acids, all of which are perilous to the already injured brain.10,27
Today, there is also increasing clinical evidence on the association between hypocapnia and adverse neurodevelopmental outcome at 18–24 months of age in infants with moderate-to-severe NE based on the retrospective analysis of large HT trials.4,5 This notion is further supported by the results of resuscitated adults13,14 and children28 following cardiac arrest, as patients with normocapnia or mild hypercapnia had better neurological outcome. However, in the absence of randomized trials of controlled normocapnia it remains unclear whether hypocapnia is truly a modifiable risk factor of unfavorable outcome.
There were several safety concerns that we tried to address in our study. First, we considered the fluctuation in PCO2 as a risk factor for developing intraparenchymal hemorrhage, because hypercapnia has been described to increase its risk in preterm infants,29 and both birth asphyxia and HT treatment could cause impaired coagulation.30,31 We performed MRI scans in all patients and found subdural, subarachnoid, and intraventricular hemorrhage (grade I) in six infants who are likely to be associated with birth trauma and found no intraparenchymal hemorrhage.
In addition, PCO2 is one of the most potent regulators of CBF with 4% increase in CBF per 1 mm Hg under normal conditions.32 In our study, ultrasound assessment of CBFVs revealed no differences in the ACA and MCA before, during, and after the intervention in the seven patients who had measurements in all three time epochs. The first three patients had no measurements before the initiation of CO2 inhalation due to technical reasons. In general, the lack of CO2 reactivity present in infants with extensive brain injury.33 However, the lack of change in CBFV in our patients is more likely related to the fact that our study was conducted in the early hours of life. In patients with NE, vascular reactivity may be transiently absent before the physiologic CO2 reactivity of cerebral vasculature appear.34,35,36
Second, it has been described that CO2 inhalation elicits a physiological response of increased minute ventilation mainly due to the increase in tidal volume.19,23,37,38 In line with this, a Canadian research group using inhaled CO2 of 0.5–1.5% via nasal prongs to prevent apnea in preterm infants noted a mild but tolerable increase in minute ventilation without any respiratory discomfort.18,20,39 Similarly, we also found an increased tidal volume during CO2 inhalation and a reduction in RR after the offset of CO2 administration. The increase in the death of respiration was clearly related to the CO2 exposition. All patients received sedato-analgesia (10 μg/kg/h morphine infusion) during the study period; therefore, we were unable to assess the possible discomfort caused by our intervention. The significance of the increased PO2 during the intervention requires further investigation.
Third, we closely monitored brain background and seizure activity of our patients. We could not find a direct relationship between CO2 inhalation and electrophysiological brain activity in our small clinical trial, although our study was not designed and lacked power to systematically assess seizure activity.
The rate of normal outcome and moderate disabilities in both groups were similar to the rate reported in the literature.24
Our study has several limitations that should be taken into consideration. First, this was a small pilot study using a historical control group, which limits powerful efficacy analysis. Also, p value should be interpreted carefully within the context of the small sample size. Second, continuous monitoring of PCO2 was not feasible during the study because of current technical limitations. However, we performed frequent arterial blood gas sampling. Transcutaneous CO2 monitoring has not been tested systematically under HT treatment. Although end-tidal CO2 monitoring has become available recently, its precision is in question.40 Furthermore, owing to the gas flow turbulence in patient circuits, the exhaled gas can be diluted with the CO2-enriched inhaled gas mixture prior to reaching the sampling port. Another limitation of our study that the tidal volumes were collected manually throughout the study period, allowing for safety analysis of respiratory parameters. Of note, the manual data collection could lead to an observational bias. This pilot trial was an initial step to explore a novel intervention and identify the modifications that are needed for a larger randomized trial to test the efficacy of controlled normocapnia on long-term neurodevelopmental outcomes.
Despite the limitations of the present trial, we suggest that controlled normocapnia with the inhalation of 5% CO2 could be a reasonable approach to enhance hypothermic neuroprotection.
Conclusion
Inhaled 5% CO2 administration is a physiologically plausible and straightforward intervention for correcting hypocapnia. Further studies are warranted to test the efficacy of CO2 inhalation in providing better neurodevelopmental outcome in asphyxiated neonates treated with HT.
References
Lawn, J. E., Kerber, K., Enweronu-Laryea, C. & Cousens, S. 3.6 million neonatal deaths-what is progressing and what is not? Semin. Perinatol. 34, 371–386 (2010).
Shankaran, S. et al. Childhood outcomes after hypothermia for neonatal encephalopathy. N. Engl. J. Med. 366, 2085–2092 (2012).
Edwards, A. D. et al. Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta-analysis of trial data. BMJ 340, c363 (2010).
Pappas, A. et al. Hypocarbia and adverse outcome in neonatal hypoxic-ischemic encephalopathy. J. Pediatrics 158, 752–8 e1 (2011).
Lingappan, K., Kaiser, J. R., Srinivasan, C. & Gunn, A. J. Relationship between PCO2 and unfavorable outcome in infants with moderate-to-severe hypoxic ischemic encephalopathy. Pediatr. Res. 80, 204–208 (2016).
Klinger, G., Beyene, J., Shah, P. & Perlman, M. Do hyperoxaemia and hypocapnia add to the risk of brain injury after intrapartum asphyxia? Arch. Dis. Child. Fetal Neonatal Ed. 90, F49–F52 (2005).
Nadeem, M., Murray, D., Boylan, G., Dempsey, E. M. & Ryan, C. A. Blood carbon dioxide levels and adverse outcome in neonatal hypoxic-ischemic encephalopathy. Am. J. Perinatol. 27, 361–365 (2010).
Szakmar, E. et al. Asphyxiated neonates who received active therapeutic hypothermia during transport had higher rates of hypocapnia than controls. Acta Paediatr. 107, 1902–1908 (2018).
Lasso Pirot, A., Fritz, K. I., Ashraf, Q. M., Mishra, O. P. & Delivoria-Papadopoulos, M. Effects of severe hypocapnia on expression of bax and bcl-2 proteins, DNA fragmentation, and membrane peroxidation products in cerebral cortical mitochondria of newborn piglets. Neonatology 91, 20–27 (2007).
Vannucci, R. C., Towfighi, J., Heitjan, D. F. & Brucklacher, R. M. Carbon dioxide protects the perinatal brain from hypoxic-ischemic damage: an experimental study in the immature rat. Pediatrics 95, 868–874 (1995).
Greisen, G., Munck, H. & Lou, H. Severe hypocarbia in preterm infants and neurodevelopmental deficit. Acta Paediatr. Scand. 76, 401–404 (1987).
Calvert, S. A., Hoskins, E. M., Fong, K. W. & Forsyth, S. C. Etiological factors associated with the development of periventricular leukomalacia. Acta Paediatr. Scand. 76, 254–259 (1987).
Vaahersalo, J. et al. Arterial blood gas tensions after resuscitation from out-of-hospital cardiac arrest: associations with long-term neurologic outcome. Crit. Care Med. 42, 1463–1470 (2014).
Schneider, A. G. et al. Arterial carbon dioxide tension and outcome in patients admitted to the intensive care unit after cardiac arrest. Resuscitation 84, 927–934 (2013).
Laffey, J. G. & Kavanagh, B. P. Hypocapnia. N. Engl. J. Med. 347, 43–53 (2002).
Kraut, J. A. & Madias, N. E. Metabolic acidosis: pathophysiology, diagnosis and management. Nat. Rev. Nephrol. 6, 274–285 (2010).
Zanelli, S., Buck, M. & Fairchild, K. Physiologic and pharmacologic considerations for hypothermia therapy in neonates. J. Perinatol. 31, 377–386 (2011).
Al-Saif, S. et al. A randomized controlled trial of theophylline versus CO2 inhalation for treating apnea of prematurity. J. Pediatrics 153, 513–518 (2008).
Alvaro, R. E. et al. A developmental study of the dose-response curve of the respiratory sensory reflex. Am. Rev. Respir. Dis. 148, 1013–1017 (1993).
Alvaro, R. E. et al. CO(2) inhalation as a treatment for apnea of prematurity: a randomized double-blind controlled trial. J. Pediatrics 160, 252–7 e1 (2012).
Forsyth, R., Martland, T., Lai, M., Vadlamani, G. & Hogan, V. 5% Carbon dioxide is safe but of limited efficacy as a treatment for paediatric non-convulsive status epilepticus: an open label observational study. Eur. J. Paediatr. Neurol. 20, 560–565 (2016).
Ohlraun, S. et al. CARbon DIoxide for the treatment of Febrile seizures: rationale, feasibility, and design of the CARDIF-study. J. Transl. Med. 11, 157 (2013).
Rigatto, H., Brady, J. P., de la Torre & Verduzco, R. Chemoreceptor reflexes in preterm infants: II. The effect of gestational and postnatal age on the ventilatory response to inhaled carbon dioxide. Pediatrics 55, 614–620 (1975).
Azzopardi, D. V. et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N. Engl. J. Med. 361, 1349–1358 (2009).
Ashwood, E. R., Kost, G. & Kenny, M. Temperature correction of blood-gas and pH measurements. Clin. Chem. 29, 1877–1885 (1983).
Thompson, C. M. et al. The value of a scoring system for hypoxic ischaemic encephalopathy in predicting neurodevelopmental outcome. Acta Paediatr. 86, 757–761 (1997).
Curley, G., Laffey J. G. & Kavanagh B. P. Bench-to-bedside review: carbon dioxide. Crit Care 14, 220 (2010).
Del Castillo, J. et al. Hyperoxia, hypocapnia and hypercapnia as outcome factors after cardiac arrest in children. Resuscitation 83, 1456–1461 (2012).
Kaiser, J. R., Gauss, C. H., Pont, M. M. & Williams, D. K. Hypercapnia during the first 3 days of life is associated with severe intraventricular hemorrhage in very low birth weight infants. J. Perinatol. 26, 279–285 (2006).
Gupta, S. N., Kechli, A. M. & Kanamalla, U. S. Intracranial hemorrhage in term newborns: management and outcomes. Pediatr. Neurol. 40, 1–12 (2009).
Forman, K. R. et al. Coagulopathy in newborns with hypoxic ischemic encephalopathy (HIE) treated with therapeutic hypothermia: a retrospective case-control study. BMC Pediatr. 14, 277 (2014).
Greisen, G. Autoregulation of cerebral blood flow in newborn babies. Early Hum. Dev. 81, 423–428 (2005).
Pryds, O., Greisen, G., Lou, H. & Friis-Hansen, B. Vasoparalysis associated with brain damage in asphyxiated term infants. J. Pediatrics 117, 119–125 (1990).
Haaland, K., Karlsson, B., Skovlund, E., Lagercrantz, H. & Thoresen, M. Postnatal development of the cerebral blood flow velocity response to changes in CO2 and mean arterial blood pressure in the piglet. Acta Paediatr. 84, 1414–20. (1995).
Noori, S., Anderson, M., Soleymani, S. & Seri, I. Effect of carbon dioxide on cerebral blood flow velocity in preterm infants during postnatal transition. Acta Paediatr. 103, e334–e339 (2014).
Pryds, O., Greisen, G., Lou, H. & Friis-Hansen, B. Heterogeneity of cerebral vasoreactivity in preterm infants supported by mechanical ventilation. J. Pediatrics 115, 638–645 (1989).
Berger, A. J., Mitchell, R. A. & Severinghaus, J. W. Regulation of respiration (first of three parts). N. Engl. J. Med. 297, 92–97 (1977).
Cross, K. W., Hooper, J. M. & Oppe, T. E. The effect of inhalation of carbon dioxide in air on the respiration of the full-term and premature infant. J. Physiol. 122, 264–273 (1953).
Al-Aif, S. et al. Inhalation of low (0.5–1.5%) CO2 as a potential treatment for apnea of prematurity. Semin. Perinatol. 25, 100–106 (2001).
Tingay, D. G., Stewart, M. J. & Morley, C. J. Monitoring of end tidal carbon dioxide and transcutaneous carbon dioxide during neonatal transport. Arch. Dis. Child. Fetal Neonatal Ed. 90, F523–F526 (2005).
Acknowledgements
We acknowledge the important contribution of Professor Dr. Istvan Seri, Professor of Paediatrics, USC Keck School of Medicine, Los Angeles, CA in discussing the study design and reviewing the paper. We thank Laszlo Szakacs, Planimeter Statistics Ltd., Budapest, Hungary for expert help with data management and statistical analysis. We also thank the medical and nursing team at the NICU of the Semmelweis University, 1st Department of Paediatrics for the professional care and study management. A.J. was supported by the Hungarian Academy of Science, Premium Postdoctoral Fellowship (PPD460004). The funding agency had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Author information
Authors and Affiliations
Contributions
E.S. contributed to the study design, recruited participants, collected the data, carried out the initial analyses, interpreted the data, wrote the initial manuscript draft, and reviewed and revised the manuscript. K.K. helped to collect and analyze the data and to write the manuscript draft. U.M. recruited participants, collected the data, analyzed the aEEG traces, and edited the manuscript. G. Bokodi and C.A. recruited patients, made significant contribution to the interpretation of data, and revised the manuscript. A.L. analyzed MRI studies and edited and revised the manuscript. A.J.S. and G. Belteki supervised the interpretation of data and revised the manuscript for important intellectual content. M.S. and A.J. conceptualized and designed the study, supervised all aspects, and critically reviewed the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Szakmar, E., Kovacs, K., Meder, U. et al. Neonatal encephalopathy therapy optimization for better neuroprotection with inhalation of CO2: the HENRIC feasibility and safety trial. Pediatr Res 87, 1025–1032 (2020). https://doi.org/10.1038/s41390-019-0697-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-019-0697-9
This article is cited by
-
Carbon dioxide and MAPK signalling: towards therapy for inflammation
Cell Communication and Signaling (2023)
-
Carbon dioxide levels in neonates: what are safe parameters?
Pediatric Research (2022)
-
Hypocapnia in early hours of life is associated with brain injury in moderate to severe neonatal encephalopathy
Journal of Perinatology (2022)
-
Carbon dioxide as a drug in neonatology
Pediatric Research (2021)