Abstract
Background
Asphyxia of newborns is a severe and frequent challenge of the peri- and postnatal period.
Methods
Forty-four neonatal piglets underwent asphyxia and hemorrhage (AH), followed by resuscitation with blood or crystalloid transfusion. In this study, 15 piglets (blood n = 9, NaCl n = 6, mean age 31 h) were randomly chosen. Four hours after return of spontaneous circulation, heart tissue and blood were collected. Analyses of heart fatty acid binding protein (HFABP), cardiac troponin I (TnI) levels, and activation of the complement system were performed. Histological staining for connexin 43 (Cx43) and complement C5a receptor 1 (C5aR1) was performed.
Results
Following AH, systemic elevation of cardiac TnI and HFABP revealed cardiac damage in both groups. Systemic activation of the complement system and the appearance of extracellular histones in plasma of the blood transfusion group were observed. The Cx43 was translocated from the intercalated discs to the cytosol after AH. Cardiac glycogen concentration was reduced in both groups. A significant reduction of C5aR1 in the left ventricle and a significant elevation of the heart injury score were investigated after blood transfusion.
Conclusion
AH leads to alteration of the heart, particularly in Cx43 and glycogen reserves, as well as local inflammation.
Similar content being viewed by others
Introduction
Asphyxia is a severe challenge during the peri- and postnatal period, occurring in 2 of 1000 births.1 It is defined as a severe disturbance of the oxygen supply to the fetus2 with an intrapartum pH of <7.00 and a base deficit >−12 mmol/l.3 In particular, the cardiac consequences of asphyxia are crucial, because impairment of the heart is linked to prolonged organ hypoperfusion. Ventricular diastolic dysfunction was present in more than 50% of asphyxiated neonates.4 Furthermore, well-known cardiovascular consequences of asphyxia include tricuspid valve and mitral valve regurgitation, pulmonary hypertension, and transient myocardial ischemia.4 Human infants, who did not survive asphyxia, presented ventricular dilatation and hypertrophy, as well as papillary muscle necrosis.5
Systemic elevation of cardiac troponin (>4.6 ng/ml) in newborns with perinatal asphyxia has been correlated with higher mortality.6 This is important because troponin is a classical marker of cardiomyocyte (CM) injury.7 Furthermore, preterm infants have a tenfold increase in baseline TnT levels compared to term newborns, which was associated with cardiac dysfunction in the preterm group.8 In neonatal respiratory distress syndrome and asphyxia, elevated troponin levels were correlated with myocardial dysfunction assessed by echocardiography.8 Experimentally, in newborn pigs after asphyxia, a reduction of cardiac myosin light chain 1 and an increase in nitrate levels in the heart has been described,9 which has been associated with cardiac dysfunction.10 Furthermore, alterations in the distribution and the amount of the gap junction protein connexin 43 (Cx43) and mitochondrial injury were observed after asphyxia in adult rats.11 Cx43 is the most important gap junction protein in the heart and is responsible for the electrical coupling. Cx43 is important for the myocytes to act as a functional syncytium and could be responsible for the development of arrythmias.12 For this reason, alterations in the Cx43 might be of interest for cardiac function after asphyxia and hemorrhage (AH).
Furthermore, the complement system is well described as a mediator of CM dysfunction, particularly in the context of septic CMs, and should be considered in cardiac analysis after trauma, sepsis, and ischemic injury.13,14 The present study aims to clarify the role of systemic activation of the complement system based on complement hemolysis CH50 measurements and systemic C5a levels, and we further determined local complement-induced changes on the heart.
The present study aims to investigate the consequences of neonatal AH in newborn pigs on cardiac damage patterns and on local and systemic inflammation. In accordance with the international guidelines of the International Liaison Committee of Resuscitation (ILCOR), asphyxia in neonates was treated with ventilation, cardiopulmonary resuscitation (CPR), epinephrine, and either with red blood cell (RBC) transfusion or saline (NaCl). Successful resuscitation was achieved in all animals independent of group assignment and without differences in the time to return of spontaneous circulation (ROSC) between crystalloid and early transfusion.15 The current report aims to clarify whether cardiac inflammation, complement activation, and gap junction alterations may contribute to the development of cardiac dysfunction after asphyxia, cardiac arrest, and hemorrhage in newborn pigs.
Materials and methods
Animals and anesthesia
In total, 44 piglets (mean age: 32 h, weighing 1.220 (1.060–1.495) kg) underwent AH, and were then randomized into two therapy groups either with NaCl volume expansion or by blood re-transfusion (RBC). Six animals underwent a sham procedure, which included anesthesia and instrumentation but without AH, followed by an observation period of 4 h. For the purpose of the present analysis, 21 animals (9 RBC, 6 NaCl, and 6 sham) were blindly chosen and randomly assigned until at least n = 6 for each group were attained. There were no significant differences in age, weight, blood loss, or re-transfusion volume between the individual groups.
All procedures conformed to the Society of Laboratory Animal Science as well as the National Animal Welfare Law and were approved by the responsible government authority (Regierungspräsidium Tübingen, Germany; TV-Nos: 1262 and 1320) in accordance with the guidelines of the Federation of European Laboratory Animal Science Association as described previously in ref. 15 Mendler et al.15 recently observed that early blood transfusion compared to crystalloid transfusion did not reduce the time to ROSC. Hemodynamic parameters after ROSC were similar, except that the mean arterial blood pressure was significantly higher in the NaCl group. In the post-resuscitation period, there were no differences between the groups regarding blood gases or metabolic and hemodynamic parameters. Detailed ventilatory parameters were published elsewhere.15
Anesthesia and preparation
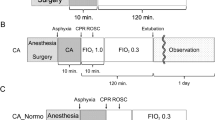
Animals were anesthetized with propofol and fentanyl and intubated orotracheally. Ventilation was conducted under controlled pressure with a FiO2 of 0.30, a positive end-expiratory pressure of 5 cmH2,O and a peak inspiratory pressure (PIP) of 15 cmH2O, which resulted in a mean Vt of 7.5 ml/kg, inspiratory time of 0.4 s, and an initial ventilation rate of 20 min−1. The ventilation frequency was adapted to a PaCO2 of 34–45 (normocapnia). The temperature was maintained between 39.0 and 39.5 °C.
A double-lumen arterial line was inserted into the right femoral artery for continuous blood pressure measurements and to obtain arterial blood gas analyses and blood samples. Additionally, a double-lumen venous line was placed into the right femoral vein for central venous pressure (CVP) measurements, intravenous drug application, and maintenance of fluids.
Asphyxia and resuscitation
After anesthesia, the FiO2 decreased to 0.21, and after 15 min, baseline blood samples were collected. Hypoxia was induced by a reduction of the FiO2 to 0.08 and simultaneous exposure to hypercapnia (FiCO2 = 0.07). The ventilation frequency was reduced stepwise every 10 min by 10 min−1 to induce alveolar hypoventilation. When a pH of 7.0 was reached, alternatively after 12 min, hypovolemia was induced by continuous and standardized blood removal (2 ml/kg/min) from the arterial line, using a negative pressure laboratory pump (Syringe pump LA-160, Landgraf Laborsysteme, Langenhagen, Germany). Blood volume depletion was continued until cardiac arrest occurred. A cardiovascular arrest was defined as the loss of pulse (blood pressure <10 mmHg) and regular ECG activity (asystole).
Resuscitation was performed according to the ILCOR guidelines. Respiratory support was discontinued for 30 s, mimicking the period of the initial steps of neonatal resuscitation. After 30 s of adequate respiratory support, CPR was initiated (PIP 25 cmH2O). Three chest compressions performed with a two-thumb method were interrupted by one inflation. During resuscitation, the tidal volume in the RBC group was 16.1 ml/kg and in the NaCl animals it was 19.6 ml/kg.15
Success of the resuscitation was evaluated every 30 s. Ninety seconds after cardiac arrest, the FiO2 was increased to 1.0 and epinephrine (20 µg/kg) was given. CPR was continued. Following a further 30 s of resuscitation, pigs received an infusion of 10 ml/kg over 2 min of NaCl 0.9% (n = 6) or a re-transfusion of withdrawn blood (n = 9). Re-transfusion was terminated when the ROSC was reached. The re-transfusion volume is presented in Fig. 2d. The return to spontaneous circulation was defined as a heart rate of >60 b.p.m. (electrocardiogram (ECG)) and a visible arterial blood pressure curve.
Follow-up and euthanasia
Hemodynamic parameters were monitored continuously. During the post-resuscitation period, the Vt in the RBC group was 8.2 ml/kg and in the NaCl animals 8.7 ml/kg,15 with a mean PIP of 15 cmH2O. The observation period was terminated after 4 h by an intravenous overdose of potassium chloride, which induced a cardiovascular arrest.
Sample collection
Serum and plasma samples were collected at baseline and 4 h after ROSC and kept on ice. Following centrifugation (800 × g for 5 min at 4 °C and 13,000 × g for 3 min), serum and EDTA-plasma were removed and stored at −80 °C until analysis. Heart tissue samples of left ventricles were obtained 4 h after resuscitation and either fixed with 4% formalin followed by embedding in paraffin or quick-frozen in liquid nitrogen for storage at −80 °C until analysis. Pericardial effusion, when available, was aspirated and after centrifugation (800 × g for 5 min) the supernatant was collected and stored at −80 °C.
Transthoracic echocardiography in piglets
Echocardiograms were performed according to the recommendations using a standard cardiac ultrasound machine (M-Turbo©, FujiFilm SonoSite BV, Amsterdam, The Netherlands). Following the acquisition of M-mode images in the parasternal long axis, systolic and diastolic parameters were measured. Serial imaging was performed at baseline and 2 and 4 h after ROSC. The following data were determined and presented in Fig. 1: stroke volume (SV), intraventricular septum in diastole (IVSd), intraventricular septum in systole (IVSs), left ventricular internal diameter at end diastole (LVIDd), left ventricular internal diameter at end systole (LVIDs), wall of the left ventricle during diastole (LVPWd), and wall of the left ventricle during systole (LVPWs), as well as the ejection fraction (EF), cardiac output (CO), and shortening fraction (SF).
Cardiac function after asphyxia and hemorrhage. a Stroke volume (SV) in ml measured by echocardiography at baseline (BL) and 2 and 4 h after return of spontaneous circulation (ROSC) with either blood transfusion (RBC) or saline (NaCl). b Heart rate in b.p.m. c Intraventricular septum in diastole (IVSd) in cm. d Intraventricular septum in systole (IVSs) in cm. e.Left ventricular internal diameter at end diastole (LVIDd) in cm. f Left ventricular internal diameter at end systole (LVIDs) in cm. g Wall of the left ventricle during diastole (LVPWd) in cm measured by echocardiography at BL and 2 and 4 h after ROSC with either RBC or NaCl. h Wall of the left ventricle during systole (LVPWs) in cm. i Cardiac output (CO) in l/min at BL and 2 and 4 h after asphyxia and hemorrhage (AH). j Shortening fraction (SF) in % at BL and 2 and 4 h after AH. k Ejection fraction (EF) in %. *p < 0.05
Histone, troponin I, HFABP, and Cx43
Histones in plasma were measured by using a Cell Death Detection ELISA kit (Hoffmann-La Roche, Indianapolis, IN). A histone mixture (containing H1, H2A, H2B, H3, and H4) (Sigma, St. Louis, MO) was used to establish a standard curve.16,17 The cardiac troponin I (TnI) concentration in serum samples was determined using the Ultrasensitive Pig Troponin I ELISA kit (Life Diagnostics, West Chester, PA) and heart fatty acid binding protein (HFABP) was determined using a Pig Cardiac Fatty Acid Binding Protein ELISA kit (Life Diagnostics, West Chester, PA). All results were correlated with the serum or plasma protein concentration to avoid diluting effects by different volume replacements. The total protein amount was determined by using the Pierce BCA Protein Assay kit (Thermo Fisher Scientific, Waltham, MA). All assays as well as the analysis and scoring of stained sections were performed blindly.
C5a-ELISA
Porcine C5a concentrations in left ventricle homogenates were analyzed using a porcine complement component 5a ELISA kit (Reddotbiotech, Kelowna, Canada). Results were correlated with the respective protein concentration.
CH50
The classical complement pathway activity was analyzed in serum samples by measuring the complement hemolytic activity (CH50). Briefly, sensitized sheep RBCs (Complement Tech, Tyler, TX) were exposed to a serial dilution of serum samples after asphyxia (for 60 min). Absorbance of the supernatant was measured at 541 nm and the serum concentration of 50% hemolysis (CH50) was determined.
Western blotting
Left ventricular tissue obtained 4 h after resuscitation or sham procedure was homogenized and lysed using RIPA Lysis Buffer (Thermo Fisher, Rockford, IL) containing complete Mini-protease inhibitor and PhosSTOP protease inhibitor cocktail (Roche, Indianapolis, IN). Protein concentrations were determined in homogenates. The samples were loaded onto a 4–20% Mini-Protean® TGX™ precasted gel (Bio-Rad Laboratories, Munich, Germany). Proteins were transferred with a Trans-Blot Turbo Transfer System (Bio-Rad). The blots were incubated with a Cx43 antibody (Cell Signaling, Danvers, MA). Horse radish peroxidase-anti-rabbit antibody (Cell Signaling Technology, Danvers, MA) was used as the secondary antibody. The blots were analyzed using the ChemiDoc (Bio-Rad) and the Image Lab Software (Version 5.2, Bio-Rad).
Determination of RNA expression of myoglobin, HFABP, IL-8, IL-6, C5aR1 NLRP3, and IL-1β in pig left ventricle homogenates by real-time quantitative PCR analysis
Total RNA was isolated from pig heart homogenates using TRIZOL® (Life Technologies). Complementary DNA (cDNA) was obtained using the superscript and then amplified using SYBR®Green (Life Technologies). Amplification was performed using the Quant Studio 3 (Applied Biosystems, Waltham, MA). Messenger RNA (mRNA) expression of the respective genes was normalized to glyceraldehyde 3-phosphate dehydrogenase expression. Calculation of the relative quantitative mRNA expression was performed with the cycle threshold methodΔΔCt algorithm. The used primers are listed in Supplement Fig. S2a.
Immunohistochemistry (C5aR1, Cx43, nitrotyrosin); H&E staining, PAS staining, and TUNEL
Formalin-fixed and paraffin-embedded hearts were used. To stain for C5aR1, polyclonal rabbit anti-pig CD88/C5aR1 (Acris Antibodies, Herford, Germany) was utilized. For Cx43 staining, rabbit anti-pig Cx43 (Cell Signaling Technology, Danvers, MA) was used. Nitrotyrosine was stained using anti-nitrotyrosine antibody (Merck Millipore, Darmstadt, Germany). Dako REALTM Detection System (Dako, Glostrup, Denmark) constituted the secondary staining system. The Fast hematoxylin and eosin (HE) staining kit (Morphisto, Frankfurt am Main, Germany) was used. To evaluate histomorphological changes, H&E-stained sections were scored for (1) apoptosis, (2) contraction band necrosis, (3) neutrophilic infiltration, (4) intramuscular bleeding, (5) rupture, (6) edema, and (7) ischemia. Scoring was conducted as previously published.18 Periodic acid Schiff (PAS) staining was performed using the PAS staining kit (Merck Millipore, Darmstadt, Germany). Signal density was measured blindly in three randomly chosen, distinct fields of vision (×100 magnification) from each slide using an Axio ImagerM.1 microscope and the Zeiss AxioVision software 4.9 (Zeiss, Jena, Germany).
TdT-mediated dUTP-biotin nick-end labeling staining (TUNEL) staining was performed using the CFTM 488 TUNEL Apoptosis Detection kit (Biotium, Fremont, CA) and counterstained with Höchst 33342 (Sigma, Darmstadt, Germany). The positive nuclei in TUNEL staining were counted blindly and presented as the percentage of the total nuclei number.
Immunofluorescence (GLUT1, GLUT4)
Formalin-fixed and paraffin-embedded hearts were utilized in immunofluorescence (IF) staining. For glucose transporter 1 (GLUT1) and GLUT4 staining, polyclonal rabbit anti-GLUT1, and for GLUT4 staining, polyclonal rabbit anti-GLUT4 (both Abcam, Cambridge, UK), respectively, were used as the primary antibody. Alexa Flour 488-labelled goat anti-rabbit antibody (AF-488) was employed as the secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA). Imaging was performed using an Axio ImagerM.1 microscope and the Zeiss AxioVision software 4.9 (Zeiss). Fluorescence intensity evaluation was conducted blinded using the Software Image J1x.
Statistical procedures
All values are expressed as the mean ± standard error of the mean (SEM). Data were analyzed by one-way analysis of variance followed by Dunnett’s or Tukey’s multiple comparison test. A p ≤ 0.05 was considered statistically significant. GraphPad Prism 7.0 software was used for statistical analysis (GraphPad Software, Incorporated, San Diego, CA). For analysis of correlation, a two-tailed Pearson’s correlation calculation was conducted with GraphPad Prism 7.0. Results of the correlation analysis are presented in the Supplemental Fig. S1.
Results
Cardiac function after AH
We conducted echocardiography at baseline and at 2 and 4 h after AH. The SV in the RBC group was reduced after 2 and 4 h (Fig. 1a), whereas the heart rate did not show any significant changes after AH or between the subgroups (Fig. 1b). The IVSd and IVSs were increased in the RBC group after 4 h, whereas no alterations of these diameters were observed in the NaCl group (Fig. 1c, d). Furthermore, we found a significant reduction of the diameters LVIDd and LVIDs in the RBC group at 2 and 4 h after AH (Fig. 1e, f). Again, the NaCl group did not present any changes in the left ventricular diameters. The LVPWd and LVPWs 4 h after AH displayed an increase in the diameter in the blood-transfused animals (Fig. 1g, h). Therefore, we were able to detect a change in the NaCl group of the LVPWs 2 h after ROSC (Fig. 1h). We did not observe any changes in the CO (Fig. 1i), the SF (Fig. 1j), or the EF (Fig. 1k) in the asphyxia model used.
Comparison between the sham, NaCl, and RBC groups
There were no differences in the age (Fig. 2a), body weight (Fig. 2b), volume of blood loss (Fig. 2c), or re-transfused blood volume (Fig. 2d).
Comparison between the sham, saline (NaCl), and blood transfusion (red blood cell (RBC)) groups. a Age in h after birth of the sham, RBC, and NaCl animals. b Weight in kg. c Blood loss during asphyxia and hemorrhage (AH) in ml (left) and % (right). d Replaced blood volume in ml (left) and % (right). n.s. Not significant
Cardiac tissue damage after AH
The cardiac tissue of the RBC group showed a higher injury score compared to sham-treated animals (Fig. 3a). TnI was measured at baseline and 4 h after ROSC. The serum TnI concentration of both treatment groups was elevated after 4 h compared to the baseline and to the sham-treated animals (Fig. 3b). Additionally, the serum heart-type fatty acid binding protein (HFABP) was elevated 4 h after AH compared to the sham-treated animals (Fig. 3c), whereas the HFABP gene expression was tenfold higher in left ventricle tissue samples in the RBC group compared to the sham-treated animals (Fig. 3e). Myoglobin mRNA expression was increased in left ventricle tissue samples 4 h after AH-NaCl compared to that in the sham-treated animals (Fig. 3d). Six of nine animals treated with AH-RBC and six of six animals treated with AH-NaCl had a pericardial effusion in 100% of the animals, with the appearance of extracellular histones in pericardial effusion fluid (mean 119.52 µg/ml) (Fig. 3f).
Cardiac tissue damage after asphyxia and hemorrhage. a Heart injury score in hematoxylin and eosin (H&E)-stained sections of the left ventricle. b Cardiac-specific troponin I in serum. c Appearance of heart-type fatty acid binding protein (HFABP). #Differences to sham procedure were significant, p < 0.05; *differences to baseline concentrations were significant, p < 0.05. d Myoglobin mRNA expression in the left ventricle. e HFABP mRNA expression in the left ventricle. f Appearance of extracellular histones in pericardial effusion fluid. *p < 0.05
Systemic and local inflammation after AH
Plasma concentrations of extracellular histones were increased 4 h after ROSC in the RBC-treated group compared to sham-treated animals (Fig. 4a). Four hours after AH, the CH50 was tendentially reduced in the AH-RBC and AH-NaCl groups in comparison with the sham-treated animals (Fig. 4b, c). Cardiac interleukin-8 (IL-8) (Fig. 4d) and IL-6 expression (Fig. 4e) were increased in the AH-NaCl-treated animals compared to the sham procedure. The mRNA expression of the complement receptor C5aR1 was increased in left ventricle homogenates of the NaCl-treated animals compared to sham-treated animals (Fig. 4f). By contrast, immunohistochemical analysis revealed a reduction of C5aR1 protein in the left ventricle (Fig. 4g) in the RBC-treated group. Additionally, C5a was significantly elevated in left ventricle homogenates of both treatment groups (Fig. 4h).
Systemic and local inflammation after asphyxia and hemorrhage. a Appearance of extracellular histones in plasma, p < 0.05; *differences were significant to sham procedure. b Complement hemolysis (CH50) value in serum plasma. c Classical complement activation analyzed by CH50 lysis in serum, p < 0.05, #differences were significant for red blood cell (RBC) vs. sham, *differences were significant for NaCl vs. sham, $differences were significant for RBC vs. NaCl. d IL-8 mRNA expression in the left ventricles. e IL-6 mRNA expression in the left ventricles. f Changes in complement factor 5a receptor 1 (C5aR1) staining density in left ventricular tissue. g C5aR1 mRNA expression in the left ventricle. h C5a elevation in the left ventricle. *p < 0.05
Translocation of Cx43 in the left ventricle and the appearance of Cx43 in blood samples after AH
Western blot analysis showed no significant changes of the Cx43 protein amount in whole tissue homogenates after AH compared to sham-treated animals (Fig. 5a). To determine whether gap junction proteins in the heart were altered after AH, immunohistochemical staining of Cx43 gap junction protein in left ventricular tissue sections was performed. In sham-treated animals, Cx43 was properly located in the intercalated discs, whereas after AH followed by RBC or NaCl treatment, Cx43 was translocated and scattered into the cytosol (Fig. 5b).
AH induced alteration in energy metabolism, absence of apoptosis, slightly increased nitrosative stress, and reduced NLRP3 and IL-1β expression
Both treatment groups displayed a less intense PAS staining in left ventricles 4 h after ROSC compared to the sham-treated group (Fig. 6a, b). Immunohistochemical analysis of GLUT1 transporter revealed no differences between the AH and sham-treated animals (frame Fig. 6c). GLUT4 transporter expression on the surface of CMs was increased in both treatment groups (Fig. 6d).
Asphyxia and hemorrhage (AH) induced an alteration in energy metabolism, absence of apoptosis, nitrosative stress, and reduced Nod-like receptor protein 3 (NLRP3) and interleukin-1β (IL-1β) expression. a Changes in the periodic acid Schiff (PAS) of the left ventricle. b PAS staining of the left ventricle. c Immunohistochemistry staining of glucose transporter 1 (GLUT1) of the left ventricle. d Changes in GLUT4 staining of the left ventricle. e TdT-mediated dUTP-biotin nick-end labeling staining (TUNEL) staining of the left ventricle. f Changes in nitrotyrosine staining of the left ventricles. g NLRP3 mRNA expression in the left ventricles. h Decrease of IL-1β mRNA expression in the left ventricles. *p < 0.05
No differences were observed in the TUNEL staining between AH and sham-treated animals (Fig. 6e). Nitrotyrosine staining revealed a statistically nonsignificant increase in nitrosative stress in cardiac tissue in both treatment groups (Fig. 6f). mRNA expression of NLRP3 (Nod-like receptor protein 3) (Fig. 6g) and IL-1β (Fig. 6h) was reduced in both treatment groups.
Discussion
Cardiac damage in human newborns was diagnosed in 20% of subjects following severe asphyxia.19 In neonatal hypoxia in rats, inhibition of CM proliferation and decreased myocyte endowment in the developing heart was described, which was associated with impaired cardiac function in later life.20 By contrast, complete recovery of cardiac function in newborn humans after myocardial infarction was observed.21 During the first 7 days post birth, rodents were able to regenerate after myocardial injury because of CM proliferation (hyperplastic growth).22 In contrast to experimental studies in adults,23 in which apoptosis has been found to play a role in cardiac arrest and resuscitation-induced myocardial injury, in the present study TUNEL staining excluded apoptosis after AH.
In human adults following CPR, pericardial injuries, hemopericardium, and epicardial and myocardial contusions were observed.24 In our study, 84% of AH pigs displayed pericardial effusion after CPR compared to sham-treated animals without pericardial effusion. Whereas cardiac damage markers were systemically increased after 4 h in both treatment groups, differences between the two treatment groups with regard to cardiac damage occurred in terms of the heart injury score. Because cardiac damage markers were increased in both treatment groups, similarly it might also be linked to ischemia–reperfusion injury. The cardiac damage in the RBC treatment group might be further aggravated by increased systemic inflammation and augmented release of damage-associated molecular patterns, such as extracellular histones (Fig. 2a).
In our study protocol, whole blood re-transfusion was applied. Therefore, neutrophil activation because of contact to external surfaces followed by neutrophil extracellular trap (NET) formation presumably occurred, whereas in the clinical setting of AH, leukocyte-depleted irradiated erythrocyte concentrates are applied.25 This might be one limitation of the present study. NETs after trauma13 and during sepsis16 are well described. Elevation of intracellular calcium and reactive oxygen species (ROS) in CMs in the presence of extracellular histones were linked to CM dysfunction.16 The NaCl group mRNA expressions of myoglobin, Il-8, Il-6, and C5aR1 were significantly increased compared to sham-treated animals and in case of myoglobin and IL-8 also compared to the RBC group. We can only speculate when interpreting these results. Hypoxia amplifies the activity of innate immune cells26 and expression of pro-inflammatory mediators. Increased expression of myoglobin during hypoxic conditions might compensate for the loss of myoglobin.27
The increased expression of C5aR1 mRNA in the NaCl group might be somewhat compensatory (Fig. 3c, e). C5aR1 protein expression was significantly reduced in the RBC group and C5a was significantly increased in both treatment groups. The anaphylatoxin C5a was shown to be cardiodepressive by acting via the C5aR1/2 on the membrane of CMs, inducing defective CM contractility, reduced CO, and finally leading to cardiac dysfunction. Moreover, C5a triggers a massive amount of cytosolic ROS and intracellular calcium [Cai2+] in isolated rat CMs,14 whereas increased [Cai2+] affected the homeostasis and the electrophysiological functions of the CMs.14 Moreover, C5a induces defects in CMs’ contractility and relaxation by altering and disturbing their action potentials. C5aR1 expression was increased on CMs 24 h after burn injury and after cecal-ligature and puncture-induced sepsis, whereas C5aR1 was reduced after multiple trauma in pigs.13 One possible explanation for the downregulation of C5aR1 after AH in the RBC group could be an internalization of the C5aR1 triggered by C5a. Cleavage of the receptor by neutrophil serine protease would also be a possible explanation for reduced C5aR protein in cardiac tissue after AH28
TnI and HFABP were elevated after AH, indicating cardiac injury.7 Furthermore, a correlation between HFABP values 4 h after AH and the duration of asphyxia in seconds was observed (Supplemental Fig. 1c). Cardiac troponin in serum/plasma (>4.6 ng/ml) in newborns with perinatal asphyxia is a sensitive marker of mortality.6 In asphyxiated human infants, troponin release after myocardial damage was associated with a lower left ventricular output and SV.29 Although the myocardium of neonates is more resistant to oxidative stress9 and displays an extended repair mechanism compared to adults,21 ischemia based on decreased coronary perfusion account for myocardial dysfunction and reduced CO.30 The increased cardiac damage score in RBC-treated animals included edema as one parameter of cardiac damage, and therefore increased IVSs and LVPWs (Fig. 1) measured in the RBC treatment group might be indicative of a component of edema. The decrease in SV in the RBC group is compensated by elevated heart rates, leading to preserved CO. Although LVID was reduced 2 and 4 h after AH incomplete restoration of circulating blood volume through decreased preload might not be the leading mechanism since CVP has not been different in both treatment groups15
In the present study, alterations in cell–cell contacts occurred in the heart. After AH followed by CPR, translocation of the gap junction protein Cx43 occurred, whereas the total Cx43 protein was unchanged (Fig. 5). In sham animals Cx43 was located strictly at the intercalated discs, whereas after AH, Cx43 was translocated and scattered into the cytosol. Alterations of Cx43 location were previously described after trauma,13,31 and in rats after resuscitation following asphyxia-induced cardiac arrest.11 Reduction of Cx43 in the heart was associated with a reduction in the EF after ischemia and myocardial infarction in humans.32 Furthermore, endocytosis of gap junction proteins resulted in disruption of the coordinative spread of electrical activation, leading to arrhythmias and cardiac dysfunction.12 Gap junction alterations might contribute to impaired twist and untwist mechanism in neonates with AH.33,34 Further echocardiography studies are needed to evaluate LV twist und untwist rate.
In addition to structural alterations in the heart after AH, modifications in substrate metabolism were observed. In earlier studies in Langendorff-perfused piglet hearts, glucose oxidation fell during sustained low-flow ischemia, ATP was 76% of the controls, in the ischemia group coronary flow was reduced, and mechanical function fell to 20% of the control.35 It is well established that the metabolism of stressed infants rapidly depletes glucose stores.36 In the present study, stable serum glucose concentrations were maintained by repetitive measurements and systemic glucose supplementation. Nevertheless, the cardiac glycogen stores of the heart were depleted after AH in the preset study and GLUT4 was increased in both treatment groups. Ischemia in adults leads to translocation of GLUT4 from intracellular storage to the plasma membrane of CMs because of a switch of their primary substrate from fatty acids to glucose.37 It is well known that ischemia in adults leads to translocation of GLUT4 from intracellular storage to the plasma membrane of CMs because of a switch of their primary substrate from fatty acids to glucose.37 In the context of AH with reduced cardiac glycogen storage in animals with balanced systemic glucose concentration, the increased expression of GLUT4 in both treatment groups might be one compensatory mechanism to restore cardiac energy utilization and therefore improve cardiac function.
Furthermore, cardiac mitochondrial respiration is significantly decreased after cardiac arrest in rats.38 Therefore, failure of mitochondrial respiration should be considered as the leading metabolism problem.
In summary, cardiac alterations, including Cx43 translocation and glycogen depletion, as well as local inflammation, were observed after AH.
References
Gillam-Krakauer, M. & Gowen Jr., C. W. Birth asphyxia. Treasure Island: StatPearls; 2019. www.ncbi.nlm.nih.gov/pubmed/28613533
Correale, M. et al. Troponin in newborns and pediatric patients. Cardiovasc. Hematol. Agents Med. Chem. 7, 270–278 (2009).
Zupan Simunek, V. Definition of intrapartum asphyxia and effects on outcome [Definition de l’asphyxie intrapartum et consequences sur le devenir]. J. Gynecol. Obstet. Biol. Reprod. (Paris) 37(Suppl. 1), S7–S15 (2008).
Shahidi, M., Evazi, G. & Afkhamzadeh, A. Echocardiographic evaluation of cardiovascular complications after birth asphyxia in term neonates. Pak. J. Med. Sci. 33, 1220–1224 (2017).
Donnelly, W. H., Bucciarelli, R. L. & Nelson, R. M. Ischemic papillary muscle necrosis in stressed newborn infants. J. Pediatr. 96, 295–300 (1980).
Simovic, A. M. et al. The role of biochemical markers as early indicators of cardiac damage and prognostic parameters of perinatal asphyxia. Vojn. Pregl. 71, 149–155 (2014).
Mair, J. et al. How is cardiac troponin released from injured myocardium? Eur. Heart J. Acute Cardiovasc. Care https://doi.org/10.1177/2048872617748553 (2017).
El-Khuffash, A. F. & Molloy, E. J. Serum troponin in neonatal intensive care. Neonatology 94, 1–7 (2008).
Doroszko, A. et al. Cardiac dysfunction in an animal model of neonatal asphyxia is associated with increased degradation of MLC1 by MMP-2. Basic Res. Cardiol. 104, 669–679 (2009).
Doroszko, A. et al. Neonatal asphyxia induces the nitration of cardiac myosin light chain 2 that is associated with cardiac systolic dysfunction. Shock 34, 592–600 (2010).
Yu, H., Qing, H. & Lei, Z. Cytidine diphosphate choline improves the outcome of cardiac arrest vs epinephrine in rat model. Am. J. Emerg. Med. 31, 1022–1028 (2013).
Agullo-Pascual, E., Cerrone, M. & Delmar, M. Arrhythmogenic cardiomyopathy and Brugada syndrome: diseases of the connexome. FEBS Lett. 588, 1322–1330 (2014).
Kalbitz, M. et al. Cardiac depression in pigs after multiple trauma—characterization of posttraumatic structural and functional alterations. Sci. Rep. 7, 17861 (2017).
Kalbitz, M. et al. Complement destabilizes cardiomyocyte function in vivo after polymicrobial sepsis and in vitro. J. Immunol. 197, 2353–2361 (2016).
Mendler, M. R. et al. Successful resuscitation in a model of asphyxia and hemorrhage to test different volume resuscitation strategies. a study in newborn piglets after transition. Front. Pediatr. 6, 192 (2018).
Kalbitz, M. et al. Role of extracellular histones in the cardiomyopathy of sepsis. FASEB J. 29, 2185–2193 (2015).
Bosmann, M. et al. Extracellular histones are essential effectors of C5aR- and C5L2-mediated tissue damage and inflammation in acute lung injury. FASEB J. 27, 5010–5021 (2013).
Braun, C. K. et al. Early structural changes of the heart after experimental polytrauma and hemorrhagic shock. PLoS ONE 12, e0187327 (2017).
Bancalari, A. et al. Myocardial damage following neonatal severe asphyxia [Dano miocardico secundario a asfixia neonatal grave]. Rev. Chil. Pediatr. 62, 232–237 (1991).
Paradis, A. N., Gay, M. S., Wilson, C. G. & Zhang, L. Newborn hypoxia/anoxia inhibits cardiomyocyte proliferation and decreases cardiomyocyte endowment in the developing heart. Role of endothelin-1. PLoS ONE 10, e0116600 (2015).
Haubner, B. J. et al. Functional recovery of a human neonatal heart after severe myocardial infarction. Circ. Res. 118, 216–221 (2016).
Price, J. F. Unique Aspects of Heart Failure in the Neonate (Springer, London, 2011).
Xanthos, T., Vasileiou, P. V. S., Kakavas, S., Syggelou, A. & Iacovidou, N. The potential role of erythropoietin as a pleiotropic agent in post-cardiac arrest syndrome. Curr. Pharm. Des. 17, 1517–1529 (2011).
Miller, A. C., Rosati, S. F., Suffredini, A. F. & Schrump, D. S. A systematic review and pooled analysis of CPR-associated cardiovascular and thoracic injuries. Resuscitation 85, 724–731 (2014).
Zheng, L., Tian, M., Zhang, Y., Dong, P. & Yang, H. Neutrophil extracellular traps were released during intraoperative blood salvage in posterior lumbar surgery. Transfus. Apher. Sci. 57, 259–264 (2018).
Nizet, V. & Johnson, R. S. Interdependence of hypoxic and innate immune responses. Nat. Rev. Immunol. 9, 609–617 (2009).
Marlinge, M. et al. Physiological stress markers during breath-hold diving and SCUBA diving. Physiol. Rep. 7, e14033 (2019).
van den Berg, C. W. et al. Mechanism of neutrophil dysfunction: neutrophil serine proteases cleave and inactivate the C5a receptor. J. Immunol. 192, 1787–1795 (2014).
Wei, Y., Xu, J., Xu, T., Fan, J. & Tao, S. Left ventricular systolic function of newborns with asphyxia evaluated by tissue Doppler imaging. Pediatr. Cardiol. 30, 741–746 (2009).
Sehgal, A., Wong, F. & Mehta, S. Reduced cardiac output and its correlation with coronary blood flow and troponin in asphyxiated infants treated with therapeutic hypothermia. Eur. J. Pediatr. 171, 1511–1517 (2012).
Kalbitz, M. et al. Experimental blunt chest trauma-induced myocardial inflammation and alteration of gap-junction protein connexin 43. PLoS ONE 12, e0187270 (2017).
Dhein, S. Cardiac ischemia and uncoupling: gap junctions in ischemia and infarction. Adv. Cardiol. 42, 198–212 (2006).
Wang, J., Khoury, D. S., Yue, Y., Torre-Amione, G. & Nagueh, S. F. Left ventricular untwisting rate by speckle tracking echocardiography. Circulation 116, 2580–2586 (2007).
Breatnach, C. R., Levy, P. T., James, A. T., Franklin, O. & El-Khuffash, A. Novel echocardiography methods in the functional assessment of the newborn heart. Neonatology 110, 248–260 (2016).
Downing, S. E. & Chen, V. Myocardial hibernation in the ischemic neonatal heart. Circ. Res. 66, 763–772 (1990).
Saha, D. et al. Association of hypoglycemia, hypocalcemia and hypomagnesemia in neonates with perinatal asphyxia. Mymensingh Med J. 24, 244–250 (2015).
Nishino, Y. et al. Ischemic preconditioning activates AMPK in a PKC-dependent manner and induces GLUT4 up-regulation in the late phase of cardioprotection. Cardiovasc. Res. 61, 610–619 (2004).
Kim, J. et al. The responses of tissues from the brain, heart, kidney, and liver to resuscitation following prolonged cardiac arrest by examining mitochondrial respiration in rats. Oxid. Med. Cell. Longev. 2016, 7463407 (2016).
Acknowledgements
We acknowledge support from the Core Facility Konfokale und Multiphotonen Mikroskopie, University of Ulm, which is a multiuser imaging facility. This work was conducted in the framework of the CRC 1149 funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)—Project number 251293561.
Author information
Authors and Affiliations
Contributions
Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data: B.W., M.R.M., I.L., J.P., S.H., C.K.B., H.H., S.S., M.K. Drafting the article or revising it critically for important intellectual content: B.W., M.R.M., I.L., M.H.-L., H.H. M.K. Final approval of the version: B.W., M.R.M., I.L., J.P., M.H.-L., S.H., C.K.B., H.H., S.S., M.K.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Weber, B., Mendler, M.R., Lackner, I. et al. Tissue damage in the heart after cardiac arrest induced by asphyxia and hemorrhage in newborn pigs. Pediatr Res 86, 709–718 (2019). https://doi.org/10.1038/s41390-019-0505-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-019-0505-6
This article is cited by
-
Early myocardial damage (EMD) and valvular insufficiency result in impaired cardiac function after multiple trauma in pigs
Scientific Reports (2021)
-
Early myocardial damage (EMD) and valvular dysfunction after femur fracture in pigs
Scientific Reports (2021)