Abstract
BACKGROUND
To compare the ability of ventricular morphology on cranial ultrasound (CUS) versus standard clinical variables to predict the need for temporizing cerebrospinal fluid drainage in newborns with intraventricular hemorrhage (IVH).
Method
This is a retrospective study of newborns (gestational age <29 weeks) diagnosed with IVH. Clinical variables known to increase the risk for post-hemorrhagic hydrocephalus were collected. The first CUS with IVH was identified and a slice in the coronal plane was selected. The frontal horns of the lateral ventricles were manually segmented. Automated quantitative morphological features were extracted from both lateral ventricles. Predictive models of the need of temporizing intervention were compared.
Results
Sixty-two newborns met inclusion criteria. Fifteen out of the 62 had a temporizing intervention. The morphological features had a better accuracy predicting temporizing interventions when compared to clinical variables: 0.94 versus 0.85, respectively; p < 0.01 for both. By considering both morphological and clinical variables, our method predicts the need of temporizing intervention with positive and negative predictive values of 0.83 and 1, respectively, and accuracy of 0.97.
Conclusion
Early cranial ultrasound-based quantitative ventricular evaluation in premature newborns can predict the eventual use of a temporizing intervention to treat post-hemorrhagic hydrocephalus. This may be helpful for early monitoring and treatment.
Similar content being viewed by others
Introduction
Premature newborns are at an increased risk for intraventricular hemorrhage (IVH) and post-hemorrhagic hydrocephalus (PHH).1 The alteration in the drainage of the cerebrospinal fluid (CSF) can cause ventricular dilation (VD), which may or may not progress to hydrocephalus.2 PHH will lead to an ischemic injury to neighboring periventricular white matter and increased risk of cerebral palsy.3,4 Certain patients with PHH will require either a temporizing or a permanent intervention to drain CSF and decrease the intraventricular pressure.5 Currently, there are no non-invasive tools to measure the intraventricular pressure to early diagnose PHH nor are there agreed upon early predictors of later need for intervention to relieve pressure.
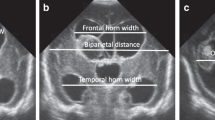
A widely available and easy to perform imaging modality used to diagnose and follow newborns with IVH is cranial ultrasound (CUS).6,7 CUS is routinely used to inspect and measure ventricular length and/or diameter as an estimate of VD.8 The size of the ventricles has been used as an indirect markers of PHH that correlates with long-term outcome.9 However, the uncertainty in the progression of VD and the lack of specificity of the measurement methods have caused delays in initiating treatment while waiting for unambiguous signs of persistent progressive hydrocephalus.10,11 In the recent ELVIS trial for early intervention for premature newborns with PHH,12 the use of a low versus high threshold of two-dimensional measurements of the ventricular size on CUS (the ventricular index (VI) and the anterior horn width) did not show a significant difference in the composite outcome of ventriculoperitoneal shunt or death. For objective evaluation, ventricular shape analysis has been applied in adult models of hydrocephalus, but its use in pediatric populations remains unclear.13
We hypothesize that the identification of the cerebral ventricular biomechanical features associated with increased intraventricular pressure in premature newborns with IVH could provide an early objective marker of PHH. A preliminary study by our group suggested that quantitative morphological shape analysis of the lateral ventricles using early CUS of premature newborns with IVH could predict the need for interventions to treat PHH.14 In this paper, we create and compare new predictive models of subsequent need for PHH treatment from ventricular morphological features seen on early CUS at the time of acute IVH, clinical variables, and a combination of the two methodologies. Our goal is to provide an early-risk stratification of newborns with IVH so that PHH treatment can be initiated earlier in those at high risk of non-resolution of their progressive ventriculomegaly.
Materials and methods
This is a retrospective study of extremely premature neonates who were admitted to our tertiary Level IV neonatal intensive care unit (NICU) at Children’s National Health System (CNHS) between the years of 2011 and 2014. Institutional Review Board approval was obtained. Inclusion criteria were: [1] <29 weeks gestational age (GA) at birth, [2] ≤1500 g birth weight (BW), [3] transferred to CNHS within 1 week after birth, [4] IVH on the CUS graded based on Papile et al.,2 and [5] survival until discharge from the NICU. Exclusion criteria were: [1] congenital brain malformation, and [2] significant subdural hemorrhage on CUS or skull fractures.
Clinical information
We reviewed the medical charts to collect the following clinical variables that have been associated with increased risk for PHH:15,16 GA, BW, gender, and head circumference at birth. The grade of IVH (1–4) was assessed by a pediatric neurologist (R.O.) on all CUS studies as a clinical variable that is associated with PHH.17 Clinical comorbidities related to prematurity were recorded, including patent ductus arteriosus requiring treatment (either medical or surgical), necrotizing enterocolitis, sepsis (confirmed with a positive blood culture), and bronchopulmonary dysplasia (the need for supplemental oxygen at 36 weeks postmenstrual age). We identified newborns that needed one or more of the procedures routinely used in our NICU to drain CSF to temporize PHH and the age at which they were performed. These procedures included: lumbar puncture, ventricular access device, and ventriculoperitoneal or ventriculoatrial shunts. The decision to perform a surgical procedure was made by the pediatric neurosurgeon based on the finding of progressive VD on weekly CUS and excessive head growth on daily head circumference. We divided the cohort into two groups: with and without temporizing intervention.
Cranial ultrasound
As part of our center’s guidelines, CUS is done on all premature infants <32 weeks GA within 24–48 h after admission. If no IVH is noted, CUS is repeated at 7, 14, and 42 days of life per the American Academy of Neurology practice parameters.7 In cases of IVH, CUS is repeated weekly to monitor for VD (as a marker for PHH), until IVH and/or VD are stable or resolved on at least two consecutive scans. If there is progression of VD on two or more CUS, a neurosurgical consultation is requested to evaluate if a temporizing or permanent surgical intervention is necessary to divert CSF. CUS are acquired using a GE LOGIQ E9 (GE Healthcare Ultrasound, Waukesha, Wisconsin) medical ultrasound system and interpreted by a pediatric radiologist.
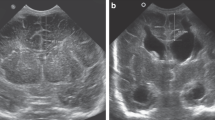
All CUS reports for every neonate were reviewed by a pediatric neurologist (R.O.) to identify the first CUS with the diagnosis of IVH and to verify the IVH grade. Then all CUS images with first diagnosis of IVH were blindly reviewed by the neurologist to select a slice in the coronal plane at the level of the foramen of Monro and manually segment the frontal horns of the lateral ventricles using the ITKSnap (http://www.itk-snap.org) medical image segmentation software. Areas of increased echogenicity, indicating hemorrhage, were segmented in the intraventricular and periventricular regions on the same CUS slice. The hemorrhage area was included as part of the clinical variables that contribute to PHH. In Fig. 1, a representative single CUS slice in a coronal view with manual segmentation of both ventricles and bleeding area are shown.
Automated morphological features
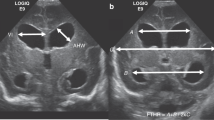
Seventy morphological features were extracted initially in an automated, unbiased manner from both ventricles on the same CUS image using in-house software tools for quantitative imaging (Fig. 2). The features could be categorized into three groups: [1] size, [2] global shape, and [3] local shape. Size features are descriptors that quantify the size of each lateral ventricle, such as area, perimeter, and the statistics of ventricle thickness in the vertical direction. Global shape features describe the geometry of the ventricles and include linear factors such as VI,18,19 lengths and ratios of the axes of the circumscribed ellipses enveloping the ventricles, and the ventricular angle.20 Intuitively, these features reflect the ballooning shape of the ventricles. Finally, local shape features measure the curvature of each ventricle boundary.21 To account for inter-patient variability in head size, these features were normalized by the size of the manually determined bounding box encompassing the cranium (i.e., maximum length of ventricles’ circumscribed ellipses, asymmetry of centroids between ventricles, and circularity of ventricles) or by the size of ventricle’s circumscribed ellipse (i.e., minimum area of the non-hemorrhagic ventricles and maximum medial length of ventricles). To account for the differences between the two lateral ventricles, predictive parameters were determined from the overall maximum, minimum, and ratio between features computed on the left and right lateral ventricles.
Examples of the morphological features considered from lateral ventricles: ventricular angle (VA), ventricular index (VI), major and minor axes (a, b) of the circumscribed ellipse, ventricle’s medial length, ventricle’s curvature, and ventricle’s thickness. The color map illustrates the curvature measure along the normal vector to the boundary
To assess the prediction ability, we compared three groups: [1] Clinical variables (seven parameters: GA, BW, gender, head circumference at birth, age in days when IVH was diagnosed, IVH grade, and normalized hemorrhage area based on skull size as segmented on the CUS image), [2] morphological features (70 parameters), and [3] the combination of clinical variables and morphological features (77 parameters). For each of the three groups, a best fit model was computed using the optimal set of predictive parameters. For the clinical variables, the optimal set was obtained by testing all possible combinations of variables using the logistic regression classifier (LOG). For the morphological features, as well as for the combination of clinical variables and morphological features, the optimal set of predictive parameters was obtained using the support vector machine (SVM) and the fast forward feature selection technique.22,23,24
SVM classifies data by defining a separating hyperplane that maximizes the distance between two classes. Specifically, given a set of training data xi,i = 1,…,n with corresponding label yi ∈ {intervention needed (yi = 1), no intervention needed (yi = −1)}, linear SVM can be determined by solving the following optimization problem25:
where w is the normal vector of the hyperplane and b is the offset of the hyperplane from the origin along the normal vector w.
LOG classifier uses a logistic function, which takes any real input x, and outputs a probability value P ∈ [0,1].25 This probability is calculated using the relationship defined below,
where a = 0.26 and c = [0.77, 0.84] are the parameters of the model.
Statistical analysis
The cohort data were used to estimate the performance of each predictive model, as described previously, using a 15-fold cross-validation method26 applied for training and testing purposes.
Each predictive model was evaluated using the sensitivity, specificity, and accuracy criteria.27 Accuracy is defined as the proportion of all outcomes that are correctly predicted. Statistical analysis was performed using the MATLAB software (Mathworks). Wilcoxon rank-sum test was used to compare continuous parametric variables between study groups, and Fisher exact test used to compare nominal and categorical variables. Continuous and categorical variables were reported as mean ± standard deviation (SD) and as percentage (%), respectively.
Results
Between 2011 and 2014, 86 premature newborns met the inclusion criteria and were diagnosed with IVH. Sixty-two (74%) survived until discharge from the NICU and were included in the analysis. Mean GA was 26 ± 2 week, mean BW was 842 ± 220 grams, and 43 (69%) were males. Mean age at first CUS was 3 ± 3 days; mean age at first CUS with IVH diagnosis was 4 ± 4 days. IVH grades on CUS when initially recognized were as follows: grade 1 (20%; n = 12), grade 2 (31%; n = 20), grade 3 (14%; n = 9), and grade 4 (35%; n = 21).
Overall, 15 (24%) infants with IVH received at least one temporizing intervention to treat PHH during their NICU course. The first intervention was either serial lumbar punctures (14/15) or placement of a ventricular access device (1/15). The first intervention was performed at a mean age of 25 ± 9 days. Clinical differences between the interventional and non-interventional groups are shown in Table 1. Of the 14 infants who had serial lumbar punctures as a first temporizing intervention, 10 needed a second intervention (8 ventricular access devices and 2 ventriculoperitoneal shunts), 3 needed no additional interventions, and 1 died later in their NICU course. The infant, who had a ventricular access device as a first intervention, rather than an initial lumbar puncture, needed a ventriculoperitoneal shunt prior to discharge.
The optimal predictive parameters of the three groups (clinical variables, morphological features, and the combination of both) are shown in Table 2. When comparing the 6 selected morphological features (out of 70) to the 2 selected clinical variables (out of 7), there was a significant improvement in the accuracy of predicting future need for a temporizing intervention (accuracy of 0.94 compared to 0.85, p value < 0.01). The optimal parameters of the group of the combination of morphological features and clinical variables (10 selected features out of 77, as shown in Table 2) increased the accuracy for treatment prediction to 0.97 with p value < 0.01 compared to the other groups. Table 3 shows the comparative performance of the optimal parameters of the three groups using sensitivity, specificity, positive predictive value, negative predictive value, area under receiver operating characteristic curve (ROC) curve, and accuracy criteria. Figure 3 illustrates the ROC curves obtained using clinical variables only, morphological features only, and the combination of clinical variables and morphological features.
Discussion
In premature newborns, the lack of a reliable measure of the ventricular changes on CUS, as a marker of progressive PHH, may cause delay in initiating necessary treatments.10
Using the initial CUS demonstrating IVH, we were able to extract morphological features of the lateral ventricles that were highly sensitive in identifying neonates who subsequently developed progressive ventriculomegaly and needed treatment for PHH. Those features were significantly more sensitive than identified clinical variables that have been associated with increased risk for PHH. Not surprisingly, the severity of the hemorrhage expressed either as the total size of the segmented hemorrhage area or as the radiological grade of IVH was the most relevant clinical variable. However, they were inferior to the morphological features of ventricular shape in differentiating newborns that would go on to have treatment for PHH.
Some of the morphological features that were very predictive include the major axes (length and width) and circularity of the ventricles (Fig. 2) and features that describe differences between the left and right lateral ventricles. All of these features are commonly described visually in the clinical practice as “ballooning” of the ventricles from hydrocephalus and asymmetry in the ventricles. We were able to quantify the subjective term “ballooning” through an objective and reproducible geometric model.
Although not significantly predictive on their own, the age at which IVH was first detected on CUS and GA in weeks had the highest predictive value of an intervention when they were in combination with the morphological features (Table 2). Of note, for each category (clinical variables only, morphological features only, or the combination of clinical variables and morphological features), the optimal set of parameters was determined independently using the machine learning classifier with feature selection. These methods select features that are not inter-correlated and collectively result in the best classifier performance. Thus the parameters selected from the combination of clinical variables and morphological features were not a concatenation of the clinical variables and morphological features when used by themselves.
Other studies have investigated the utility of volume analysis in detection of early changes in the lateral ventricles in neonate.28,29,30,31 Brann et al.31 developed a method based on the lateral ventricles volume to prospectively predict the need of intervention in premature newborns with IVH grade 3–4. They used serial measurements in order to predict the need for intervention. Kishimoto et al.30 also used a three-dimensional technology on the CUS to calculate a threshold for the ventricular volume that correlated with subsequent interventions for VD. Our approach is different since it focuses on the morphological analysis to better estimate the effect of the biomechanical forces that can affect the dimensions and shape of the ventriculomegaly due to increased intraventricular pressure. We also used the first CUS with IVH rather than serial measurements.
A method was previously reported by Mondal et al.32 who were able to identify significant ventriculomegaly using limited features for shape analysis (the anterior horn width). Our method, however, defined multiple novel features that were very sensitive in detecting particular ventricular shape associated with acute IVH that later developed into PHH. Of note, some of the features we considered included parameters that are commonly used in the evaluation of ventriculomegaly such as the VI. However, more complex features (see Table 2) were more strongly predictive of PHH.
Our study has several limitations. First, our evaluation was limited to the frontal horns of the lateral ventricles and not including the rest of the ventricular system, like the third and fourth ventricles. Second, the imaging evaluation could be affected by the imaging acquisition technique, especially that CUS imaging was performed by multiple technicians and the head position is not usually reported. Third, the evaluation was limited to the first CUS showing IVH, which could potentially continue to worsen after initial diagnosis. In addition, coronal sections were used only and the full extent of the hemorrhage may have been underestimated. The manual segmentation of the lateral ventricles was also challenging at times and needed multiple reviews, especially with IVH grade 4 where the IVH may have distorted the ventricular borders.
It is important to mention that the decision for treatment for PHH could be affected by a selection bias by the neurosurgeon, especially given the inability to measure the CSF pressure in newborns. We currently depend on multiple criteria (clinical, sonographic, and the surgeon opinion) to decide the time to perform an intervention. This may explain the delay in initiating an intervention to treat PHH in our cohort (25 ± 9 days). This variability in the time of intervention in premature newborns with PHH has been a challenge at a national and international level.10 In the study by Bassan et al.,33 early CSF external diversion (<25 days of age) was associated with a lower incidence of cerebral palsy and better cognitive outcome.33 Using our method, we were able to identify newborns who will need an intervention in the future much earlier (4 ± 4 days).
Despite these limitations, we were able to show a good accuracy of our quantitative approach in predicting surgical decision, which is usually done after the consultation of multiple services that are involved in the care of those premature newborns (neurology, neonatology, and neurosurgery) with clear agreement about treating hydrocephalus. In addition, since all our subjects were born outside of the institution, there is a potential referral bias and our cohort may have a different distribution of IVH severity and PHH than seen in the general NICU population. However, the subjects we included were admitted within a week after birth, and many of them did not have IVH on the first CUS in our institution (18/62).
In future work, we will investigate the role of CUS with quantitative imaging to predict the need for temporizing intervention even before IVH is observed.
Conclusion
CUS-based quantitative imaging for ventricular evaluation in premature newborns with post-hemorrhagic VD accurately predicts the need to perform a temporizing intervention to drain CSF at the time of the first ultrasound where intra-ventricular hemorrhage was observed. The combination of clinical variables and morphological ventricular features significantly outperformed the predictive accuracy of previously established clinical variables. Quantitative analysis of CUS could be useful in identifying the newborns with IVH who are at increased risk for complications and assure close monitoring and early treatment.
References
Radic, J. A., Vincer, M. & McNeely, P. D. Outcomes of intraventricular hemorrhage and posthemorrhagic hydrocephalus in a population-based cohort of very preterm infants born to residents of Nova Scotia from 1993 to 2010. J. Neurosurg. Pediatr. 15, 580–588 (2015).
Papile, L. A. et al. Posthemorrhagic hydrocephalus in low-birth-weight infants: treatment by serial lumbar punctures. J. Pediatr. 97, 273–277 (1980).
Del Bigio, M. R., Kanfer, J. N. & Zhang, Y. W. Myelination delay in the cerebral white matter of immature rats with kaolin-induced hydrocephalus is reversible. J. Neuropathol. Exp. Neurol. 56, 1053–1066 (1997).
Del Bigio, M. R., Wilson, M. J. & Enno, T. Chronic hydrocephalus in rats and humans: white matter loss and behavior changes. Ann. Neurol. 53, 337–346 (2003).
Strahle, J. et al. Mechanisms of hydrocephalus after neonatal and adult intraventricular hemorrhage. Transl. Stroke Res. 3(Suppl 1), 25–38 (2012).
Sarkar, S. et al. Screening cranial imaging at multiple time points improves cystic periventricular leukomalacia detection. Am. J. Perinatol. 32, 973–979 (2015).
Ment, L. R. et al. Practice parameter: neuroimaging of the neonate: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology 58, 1726–1738 (2002).
Davies, M. W. et al. Reference ranges for the linear dimensions of the intracranial ventricles in preterm neonates. Arch. Dis. Child. Fetal Neonatal Ed. 82, F218–F223 (2000).
Srinivasakumar, P. et al. Posthemorrhagic ventricular dilatation-impact on early neurodevelopmental outcome. Am. J. Perinatol. 30, 207–214 (2013).
Riva-Cambrin, J. et al. Center effect and other factors influencing temporization and shunting of cerebrospinal fluid in preterm infants with intraventricular hemorrhage. J. Neurosurg. Pediatr. 9, 473–481 (2012).
Randomised trial of early tapping in neonatal posthaemorrhagic ventricular dilatation. Ventriculomegaly Trial Group. Arch. Dis. Childhood. 65(1 Spec No), 3–10 (1990).
de Vries, L. S. et al. Treatment thresholds for intervention in posthaemorrhagic ventricular dilation: a randomised controlled trial. Arch. Dis. Child. Fetal Neonatal Ed. https://doi.org/10.1136/archdischild-2017-314206 (2018).
Momjian, S. & Bichsel, D. Nonlinear poroplastic model of ventricular dilation in hydrocephalus. J. Neurosurg. 109, 100–107 (2008).
Tabrizi, P. R. et al. Cranial ultrasound-based prediction of post hemorrhagic hydrocephalus outcome in premature neonates with intraventricular hemorrhage. IEEE Eng. Med. Biol. Soc. Annu. Conf. 2017, 169–172 (2017).
Adams-Chapman, I. et al. Neurodevelopmental outcome of extremely low birth weight infants with posthemorrhagic hydrocephalus requiring shunt insertion. Pediatrics 121, e1167–e1177 (2008).
Klinger, G. et al. Risk factors associated with post-hemorrhagic hydrocephalus among very low birth weight infants of 24-28 weeks gestation. J. Perinatol. 36, 557–563 (2016).
Radic, J. A., Vincer, M. & McNeely, P. D. Temporal trends of intraventricular hemorrhage of prematurity in Nova Scotia from 1993 to 2012. J. Neurosurg. Pediatr. 15, 573–579 (2015).
Levene, M. I. Measurement of the growth of the lateral ventricles in preterm infants with real-time ultrasound. Arch. Dis. Child. 56, 900–904 (1981).
Brouwer, M. J. et al. New reference values for the neonatal cerebral ventricles. Radiology 262, 224–233 (2012).
Fabijańska, A. et al. Assessment of hydrocephalus in children based on digital image processing and analysis. Int. J. Appl. Math. Comput. Sci. 24, 299–312 (2014).
Kovalevsky, V. Curvature in digital 2D images. Int. J. Pattern Recognit. Artif. Intell. 15, 1183–1200 (2001).
Cerrolaza, J. J. et al. Quantitative ultrasound for measuring obstructive severity in children with hydronephrosis. J. Urol. 195(Pt 1), 1093–1099 (2016).
Mladeni, D. et al. Feature selection using linear classifier weights: interaction with classification models. In Proc. 27th Annual International ACM SIGIR Conference on Research and Development in Information Retrieval 234–241 (ACM, New York, NY, 2004).
Deng, K. OMEGA: On-line Memory-based General Purpose System Classifier. PhD thesis, Carnegie Mellon Univ. (1998).
Scholkopf, B, & Smola, A. J. Learning with Kernels: Support Vector Machines, Regularization, Optimization, and Beyond (MIT Press, Cambridge, MA, 2002).
Kohavi, R. A study of cross-validation and bootstrap for accuracy estimation and model selection. In Proc. 14th International Joint Conference on Artificial Intelligence - Volume 2 1137–1143 (Morgan Kaufmann Publishers Inc., Burlington, MA, 1995).
DMW, P. Evaluation: from precision, recall and F-measure to ROC, informedness, markedness& correlation. J. Mach. Learn Technol. 2, 37–63 (2011).
Qiu, W. et al. Automatic segmentation approach to extracting neonatal cerebral ventricles from 3D ultrasound images. Med. Image Anal. 35, 181–191 (2017).
McLean, G. et al. Measurement of the lateral ventricles in the neonatal head: comparison of 2-D and 3-D techniques. Ultrasound Med. Biol. 38, 2051–2057 (2012).
Kishimoto, J. et al. Quantitative 3-D head ultrasound measurements of ventricle volume to determine thresholds for preterm neonates requiring interventional therapies following posthemorrhagic ventricle dilatation. J. Med. Imaging (Bellingham) 5, 026001 (2018).
Brann, B. St et al. Measurement of progressive cerebral ventriculomegaly in infants after grades III and IV intraventricular hemorrhages. J. Pediatr. 117, 615–621 (1990).
Mondal, P. et al. A robust method for ventriculomegaly detection from neonatal brain ultrasound images. J. Med. Syst. 36, 2817–2828 (2012).
Bassan, H. et al. Timing of external ventricular drainage and neurodevelopmental outcome in preterm infants with posthemorrhagic hydrocephalus. Eur. J. Paediatr. Neurol. 16, 662–670 (2012).
Acknowledgments
Thank you to Dr. Joseph Piatt MD from the Department of Neurosurgery at Nemours/A. I. DuPont Hospital for Children for his review of this manuscript and his valuable remarks.
Author contributions
R.O. conceptualized and designed the study, acquired the data, performed the technical analysis of the ultrasound images, and drafted the reviewed the manuscript. P.R.T. conceptualized and designed the study, acquired the data, performed the technical analysis of the ultrasound images, and drafted and reviewed the manuscript. She also performed the statistical analysis. M.G.L. helped conceptualizing and designing the study and critically reviewing the manuscript for important intellectual content and final approval of the version to be published. A.A.P. helped conceptualizing and designing the study and critically reviewing the manuscript for important intellectual content and final approval of the version to be published. J.J.C. helped conceptualizing and designing the study and reviewed the manuscript. A.M. helped conceptualizing and designing the study and critically reviewing the manuscript for important intellectual content and final approval of the version to be published. T.C. helped drafting the article and revising it critically for important intellectual content and final approval of the version to be published.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Obeid, R., Tabrizi, P.R., Mansoor, A. et al. Ventricular shape evaluation on early ultrasound predicts post-hemorrhagic hydrocephalus. Pediatr Res 85, 293–298 (2019). https://doi.org/10.1038/s41390-018-0252-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-018-0252-0