Abstract
Background
Maternal smoking, substance misuse in pregnancy and prone sleeping increase the risk of sudden infant death syndrome (SIDS). We examined the effect of maternal smoking, substance misuse and sleeping position on the newborn response to hypoxia.
Methods
Infants born between 36 and 42 weeks of gestational age underwent respiratory monitoring in the prone and supine sleeping position before and during a hypoxic challenge. Minute ventilation (MV) and end-tidal carbon dioxide (ETCO2) levels were assessed.
Results
Sixty-three infants were studied: 22 controls, 23 whose mothers smoked and 18 whose mothers substance-misused and smoked. In the supine position, baseline MV was higher and ETCO2 levels were lower in infants of substance-misusing mothers compared to controls (p = 0.015, p = 0.017, respectively). Infants of substance-misusing mothers had a lower baseline MV and higher ETCO2 levels in the prone position (p = 0.005, p = 0.004, respectively). When prone, the rate of decline in minute ventilation in response to hypoxia was greater in infants whose mothers substance-misused and smoked compared to controls (p = 0.002) and infants of smoking mothers (p = 0.016).
Conclusion
The altered response to hypoxia in the prone position of infants whose mothers substance-misused and smoked in pregnancy may explain their increased vulnerability to SIDS.
Similar content being viewed by others
Introduction
Despite the significant reduction in the number of cases of sudden infant death syndrome (SIDS) following the ‘Back to Sleep’ campaign in which parents were advised to avoid their infant-prone sleeping, there were over 2000 SIDS cases in the United States in 2012.1 Following the ‘Back to sleep’ campaign, other risk factors such as maternal smoking and maternal substance misuse became more prominent.2
A meta-analysis of 35 case–control studies estimated an approximately twofold increase in the risk of SIDS in infants of mothers who smoked in pregnancy compared to controls (odds ratio (OR) = 2.25, 95% confidence intervals (95% CI) = 2.03–2.50).3 Furthermore, the risk was dose-dependent.3 Infants of substance-misusing mothers (ISAMs) were also at increased risk of SIDS compared to controls. This risk has been reported to be from fourfold to eightfold.4,5 The effect of a combination of risk factors may be more than additive, in particular non-supine sleeping and smoking in pregnancy.6
In this study, we have tested the hypotheses that the ventilatory response to hypoxia would be dampened in infants of smoking and/or substance-misusing mothers compared to infants not exposed to either insult (controls) and any dampening would be more marked in the prone compared to the supine position.
Methods
Infants were eligible for inclusion in the study if they were born between 36 and 42 weeks of gestation at King’s College Hospital NHS Foundation Trust. The exclusion criteria were major congenital abnormalities, respiratory disease or sepsis. Approval for the study was obtained from London—Bromley Research Ethics Committee. Parents gave informed written consent for their infant to participate.
Protocol
Infants were studied prior to maternity unit discharge in the first week after birth. After a feed, the infant was placed in either the prone or supine sleeping position, the other position being studied afterwards on the same day. The order in which the positions were studied was randomised between infants. Measurements were made once the infant was in quiet sleep. Sleep state was determined by observation of the behavioural state.7 If arousal occurred, the measurement was abandoned and recommenced once the infant returned to quiet sleep.
Baseline ventilation was measured for 5 min with the infants breathing medical air from a cylinder (BOC Gases, UK). The inspired gas was then switched to a premixed gas containing 15% oxygen in nitrogen delivered from a cylinder (BOC Gases, UK). Exposure to the hypoxic gas was maintained for a further 5 min. The test was terminated if the transcutaneous oxygen saturation fell below 85%. The last minute of tidal breathing prior to switching to the hypoxic gas mix was used as a baseline value.
Equipments
The test gas was delivered using an open-circuit system via a soft latex nasal mask (Neomask, Draeger, Germany) and a pneumotachograph (Mercury F10L, G M Instruments, Kilwinning, Scotland) to measure respiratory flow. Pressure drop across the pneumotachograph was measured using a differential pressure transducer–amplifier system (Gould model 13-4615-70, Cleveland, OH, USA). The pneumotachograph had a dead space of 0.8 ml and resistance of 0.86 mmH2O/l/min (manufacturer’s data) and was connected to the nasal mask using a snugly fitting connector. A leak-free seal over the infant’s nose was achieved by gentle pressure and confirmed by the absence of any discrepancy between the inspired and expired tidal volumes on the real-time computer display. The distal end of the pneumotachograph was connected to the common port of a two-way, non-rebreathing valve which separated inspired from expired gas and ensured that the controlled mixture of gases was inspired by the infant. A constant flow of gas was delivered to the inspiratory port of the valve via a wide-bore (20 mm), low-resistance tubing eliminating any dead space. A capnograph (CO2SMO capnograph; Respironics, Chichester, UK) sampled gas continuously from the nasal mask through a fine-bore catheter at a rate of 180 ml/min. The carbon dioxide content of the sampled gas was determined by infrared spectroscopy. Respiratory flow and gas concentration were acquired and displayed in real time on a PC computer running Spectra software (Grove Medical, London, UK) with 100 Hz analogue-to-digital sampling (PCI-MIO-16XE-50, National Instruments, Austin, TX, USA). Tidal volume was obtained by digital integration of the airflow signal by the acquisition software. Minute ventilation was calculated per breath from the tidal volume trace. Oxygen saturation was measured using a pulse oximeter (Masimo rainbow SET Pulse Oximetry) attached to the foot of the infant.
Data were averaged over 10-s periods. The averaged data points were used to quantify the initial increase in ventilation in response to hypoxia and the timing and magnitude of the subsequent decline in ventilation in response to sustained hypoxia.
The responses to the hypoxic challenge were determined by
-
The time from the start of hypoxic challenge to the peak ventilatory response
-
The magnitude of the increase in minute ventilation from baseline to the peak ventilatory response
-
The magnitude of decline in minute volume from the peak to the lowest minute volume
-
The rate of decline in minute volume, calculated as the peak minute volume − lowest minute volume divided by the time from the peak to the lowest minute volume
-
The change in the oxygen saturation level from baseline to the lowest oxygen saturation level
Exposure to smoking and substance misuse
Urine samples were obtained from all infants and mothers at the time of study for cotinine analysis and assessment of maternal substance misuse. Urine was tested for cannabinoids, opiates, amphetamines, methadone, cocaine and benzodiazepines using cloned enzyme donor immunoassays (CEDIA).
Data collection
Data were gathered from the medical records: gestational age at birth, birth weight, mode of delivery, Apgar score, ethnicity, parity, maternal reported smoking and substance misuse during pregnancy. Individual birth weight centiles were calculated using the data that took into account gestational age, sex and birth weight8 via an online calculator.9
Analysis
Recruited infants were divided into three groups:
-
Infants of mothers who neither smoked nor misused substances during pregnancy (controls)
-
Infants of mothers who smoked, but did not misuse substances during pregnancy
-
Infants of mothers who misused substances during pregnancy (see Results, all mothers who substance-misused and smoked)
Statistical analyses
Differences in baseline characteristics between the three groups were assessed for statistical significance using the Kruskal–Wallis analysis of rank test or the chi-square test as appropriate. Differences in the responses to the hypoxic challenge between groups were assessed using regression analysis. Data were transformed as necessary using a square root or logarithmic transformation to meet regression assumptions. Adjustment was made for baseline differences in birthweight, gestational age and postnatal age at study by fitting the variables as covariates. The results are presented as unadjusted and adjusted arithmetic means or geometric means. Adjusted means are the marginal estimates set to the mean value of the covariates. Comparisons between prone and sleeping positions were made using the paired-samples t test.
Analyses were conducted using SPSS Version 22 (SPSS Inc., Chicago, IL, USA).
Sample size
Recruitment of 20 infants into each of the three groups allowed detection of a difference of one standard deviation in each outcome between the groups with 80% power at the 5% level. That magnitude of difference had been detected in the ventilatory response to added dead space between newborns of smoking and nonsmoking mothers.10
Results
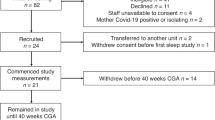
Ninety-one infants were recruited to the study. Twenty-eight infants did not complete the study protocol as the infants did not sleep (n = 22) or were discharged before the study could be performed (n = 6). There were no significant differences between those who were and were not studied with respect to gestational age, birth weight, birth weight centile or sex (Table 1).
Sixty-three infants were studied in the newborn period. There were no significant differences between the three groups with regard to gestational age, birth weight, birth weight centile, sex and age of study (Table 2).
Urine results
The urine drug screen was positive for all mothers in the substance-misusing group, and negative for all mothers and infants in the control and smoking group. All urine results for those mothers who substance-misused showed that they had smoked antenatally. Two infants in the substance-misusing group had reported maternal use of amphetamines, benzodiazepines and 3,4-methylenedioxymethamphetamine (ecstasy), but these were not detected on urine screen (Table 3). All urine samples of control mothers and infants were negative for cotinine. All maternal urines in both the smoking group and substance-misusing group were positive for cotinine. Urine from two infants in the smoking group and two in the substance-misusing group was negative for cotinine.
Four infants from the substance-misusing group subsequently required treatment for withdrawal with oral morphine, but the assessments had been made prior to commencing treatment.
Results in the supine position
In the supine position, at baseline while breathing air, the tidal volume, respiratory rate and minute volume differed significantly between the three groups. Tidal volume was lower (p = 0.011) and the respiratory rate and minute ventilation were higher in the substance-misusing group compared to controls (p < 0.001, p = 0.008, respectively). Baseline tidal volume was also lower and the respiratory rate was higher in infants of substance-misusing mothers compared to infants of smoking mothers (p = 0.002, p = 0.017, respectively) (Table 4). There were, however, no significant differences between the groups in any of the measures of the hypoxic ventilatory response (Table 5).
Results in the prone position
There were no significant differences in the baseline results of tidal volume, respiratory rate and minute volume between the three groups when studied in the prone position (Table 6). There were, however, statistically significant differences in the rate of decline in minute volume during hypoxia across the three groups (p = 0.002) (Fig. 1). A greater rate of decline was observed in the infants of substance-misusing mothers compared to the controls (p = 0.002) and infants of smoking mothers (p = 0.016). These differences remained significant after adjusting for gestation at birth, postnatal age and birth weight (Table 7).
Comparison of the results in the supine and prone positions
In the controls, sleeping position had no effect on baseline ventilatory variables. The rate of decline in minute volume during hypoxia, however, was greater in the supine compared to the prone position (p = 0.02) (Table 8). In the smoking group, at baseline, the respiratory rate was significantly lower in the prone compared to the supine position (Fig. 2), but sleeping position had no significant effect on the ventilatory response to hypoxia (Table 9). In the substance-misusing group, prone sleeping was associated with a lower respiratory rate (p = 0.001) (Fig. 2) and minute volume (Fig. 3) (p = 0.005) and a trend towards a higher end-tidal CO2 compared to the supine position (Table 10). There were no significant differences in the response to hypoxia between sleeping position.
Discussion
We have demonstrated a significantly greater rate of decline in minute ventilation during the hypoxic challenge in the prone position in infants of mothers who had misused substances and smoked during pregnancy compared to those whose mothers had smoked or whose mothers neither substance misused nor smoked.
Maternal cocaine use in pregnancy can alter placental blood flow inducing intermittent hypoxia.11 Furthermore, maternal opiate use can cause intermittent hypoxia via direct effects on maternal respiratory control.12 Fourteen adult methadone users were studied while breathing air and during hypoxic and hypercarbic challenges. The ventilatory responses to both challenges were damped, even when the adults were breathing air, and frequent oxygen desaturations occurred following methadone administration.12 Martin et al. demonstrated that chronic intermittent hypoxia in piglets increased the magnitude of this hypoxic decline.13 The decline in ventilation in response to prolonged hypoxia is centrally mediated.14 Our results then suggest that maternal substance misuse had a central effect on respiratory control. However, the effect of maternal substance misuse on the rate of hypoxic ventilatory decline was not seen in the supine position. In the supine position, respiratory drive may be maintained during hypoxia, whereas prone positioning may dampen respiratory control such that hypoxia has a significant effect on the response to hypoxia. This would be supported by our findings of differing baseline ventilation in the prone and supine position in infants of smoking and substance-misusing mothers.
We have demonstrated significant differences in baseline ventilation compared to controls in infants exposed to smoking and substance misuse during pregnancy and between the prone and supine sleeping position. Infants exposed to substance misuse during pregnancy had a significantly higher respiratory rate and lower tidal volumes in the supine position. A possible explanation for those findings is that the infants were withdrawing from substance exposure. The end-tidal CO2 was significantly lower in infants of substance-misusing mothers, suggesting that the increased minute volume and respiratory rate were not driven solely by increased metabolic demands and increased CO2 production. The findings of the differences in baseline ventilation between groups are consistent with other studies evaluating respiratory control in infants exposed to substances in utero. Glass et al.15 measured the respiratory rate and blood gases of 22 infants born to heroin-addicted mothers and compared them to unexposed controls matched by gestational age and birth weight. The heroin-exposed infants had significantly higher respiratory rates and a higher pH compared to controls. No measurements of tidal volume, however, were included. Ali et al.16 reported significantly higher minute volumes and respiratory rates in infants of substance-misusing mothers. In contrast, Wingkun et al.17 found no difference in baseline respiratory characteristics between 12 term infants born to substance-misusing mothers and 12 controls. The infants, however, were mostly studied within the first 24 h after birth and, therefore, may not have exhibited evidence of withdrawal at the time of measurement.17 Prone sleeping was associated with significantly lower respiratory rates and minute volumes and higher end-tidal CO2 levels when compared to sleeping supine in infants exposed to substance misuse in utero. The infants were studied supine and prone within the same protocol and hence their baseline results in the two positions were obtained usually less than 60 min apart. Hence, we feel that it is appropriate to compare the results to determine if there was an effect of position on tidal and minute volume. Prone compared to supine sleeping has been shown to result in a greater functional residual capacity with improved oxygenation in convalescent preterm infants.18 In 18 prematurely born infants (median gestational age 30 weeks), a stronger Hering–Breuer inflation reflex was demonstrated in the prone compared to supine position. The strength of the reflex, which is mediated by pulmonary stretch receptors, correlated strongly with the higher FRC in the prone position.19 In the prone position, we therefore hypothesise that it may exert a greater inhibitory effect on respiratory drive, hence a lower respiratory rate and minute volume in the prone position in the infants of substance-misusing mothers.
Smith et al. found no significant differences in minute volume, tidal volume or respiratory rate between the prone and supine sleeping positions in 18 convalescent preterm infants who had not been exposed to substance misuse in utero.20 This is in keeping with the results from our control infants. Ali et al. reported a significantly greater initial ventilatory increase in response to hypoxia in the supine position in infants of substance-misusing mothers compared to controls and infants of smoking mothers studied.16 No such significant differences were found in this study. It is possible that the infants we assessed had less exposure to either maternal smoking or substance misuse, as in the previous study,16 there was a significant difference in birth weight and gestation between the infants of substance-misusing, smoking and control parents which was not seen in this cohort. A comparison of urinary cotinine levels highlighted higher cotinine levels in the previous study (median 145 (range 11–8760) ng/ml vs. 130 (range 0–3240) ng/ml (p = 0.036)). Furthermore, 9 of 21 infants of substance-misusing mothers in the previous study subsequently required treatment for neonatal abstinence syndrome compared to only 4 of 17 infants in the present study.
This study had strengths and some limitations. To our knowledge, this is the first study to evaluate the combined effect of sleeping position with the antenatal risk factors for SIDS: that is, maternal smoking and substance misuse. As we were able to assess the infants prior to maternity/neonatal unit discharge, we were able to assess the effect of only antenatal exposure. The assessment of the hypoxic response quantified both the initial hypoxic ventilatory response and the later decline in ventilation. The latter component has frequently been ignored in the assessment of hypoxic responses,21,22,23 despite persisting at the high-risk age for SIDS.24 We did not rely on maternal reporting of smoking and substance misuse but assessed the results of maternal and infant urine screens. A further limitation is that we could not assess the specific effects of other drug misuse as a polydrug course.
In conclusion, infants of substance-misusing mothers who were studied in the prone position had a significantly faster decline in ventilation in response to hypoxia than infants exposed to only smoking in utero or controls. This altered ventilatory response to hypoxia may impair an infant’s ability to respond effectively to an exogenous stressor and increase the vulnerability to SIDS.
Disclaimer
The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
References
Mathews, T. J. & MacDorman, M. F. Infant mortality statistics from the 2009 period linked birth/infant death data set. Natl Vital. Stat. Rep. 61, 1–27 (2013).
Blair, P. S. et al. Major epidemiological changes in sudden infant death syndrome: a 20 year population-based study in the UK. Lancet 367, 314–319 (2006).
Zhang, K. & Wang, X. Maternal smoking and increased risk of sudden infant death syndrome: a meta-analysis. Leg. Med. 15, 115–121 (2013).
Ward, S. L. et al. Sudden infant death syndrome in infants of substance-abusing mothers. J. Pediatr. 117, 876–881 (1990).
Kandall, S. R. et al. Relationship of maternal substance abuse to subsequent sudden infant death syndrome in offspring. J. Pediatr. 123, 120–126 (1993).
Oyen, N. et al. Combined effects of sleeping position and prenatal risk factors in sudden infant death syndrome: the Nordic Epidemiological SIDS Study. Pediatrics 100, 613–621 (1997).
Prechtl, H. F. The behavioural states of the newborn infant (a review). Brain Res. 76, 185–212 (1974).
Gardosi, J. et al. An adjustable fetal weight standard. Ultrasound Obstet. Gynecol. 6, 168–174 (1995).
Gardosi J. Generic Birthweight Centile Calculator. 1.0 ed (Gestation Network, 2010).
Bhat, R. Y. et al. Dampened ventilatory response to added dead space in newborns of smoking mothers. Arch. Dis. Child. Fetal Neonatal Ed. 90, F316–F319 (2005).
Woods, J. R. Jr, Plessinger, M. A. & Clark, K. E. Effect of cocaine on uterine blood flow and fetal oxygenation. JAMA 257, 957–961 (1987).
Santiago, T. V., Pugliese, A. C. & Edelman, N. H. Control of breathing during methadone addiction. Am. J. Med. 62, 347–354 (1977).
Martin, R. J. et al. Role of inhibitory neurotransmitter interactions in the pathogenesis of neonatal apnea: implications for management. Sem. Perinatol. 28, 273–278 (2004).
Dawes, G. S. et al. Breathing in fetal lambs: the effect of brain stem section. J. Physiol. 335, 535–553 (1983).
Glass, L. et al. Effect of heroin withdrawal on respiratory rate and acid-base status in the newborn. N. Engl. J. Med. 286, 746–748 (1972).
Ali, K. et al. Antenatal substance misuse and smoking and newborn hypoxic challenge response. Arch. Dis. Child. Fetal Neonatal Ed. 101, F143–F148 (2016).
Wingkun, J. G. et al. Decreased carbon dioxide sensitivity in infants of substance-abusing mothers. Pediatrics 95, 864–867 (1995).
Kassim, Z. et al. Sleeping position, oxygen saturation and lung volume in convalescent, prematurely born infants. Arch. Dis. Child. Fetal Neonatal Ed. 92, F347–F350 (2007).
Landolfo, F. et al. Hering-Breuer reflex, lung volume and position in prematurely born infants. Pediatr. Pulmonol. 43, 767–771 (2008).
Smith, A. P. et al. The effects of sleeping position on ventilatory responses to carbon dioxide in premature infants. Thorax 65, 824–828 (2010).
Lewis, K. W. & Bosque, E. M. Deficient hypoxia awakening response in infants of smoking mothers: possible relationship to sudden infant death syndrome. J. Pediatr. 127, 691–699 (1995).
Ward, S. L. et al. Responses to hypoxia and hypercapnia in infants of substance-abusing mothers. J. Pediatr. 121, 704–709 (1992).
Ueda, Y. et al. Control of breathing in infants born to smoking mothers. J. Pediatr. 135, 226–232 (1999).
Cohen, G., Malcolm, G. & Henderson-Smart, D. Ventilatory response of the newborn infant to mild hypoxia. Pediatr. Pulmonol. 24, 163–172 (1997).
Acknowledgements
The research was supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St. Thomas’ NHS Foundation Trust and King’s College London.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rossor, T., Ali, K., Bhat, R. et al. The effects of sleeping position, maternal smoking and substance misuse on the ventilatory response to hypoxia in the newborn period. Pediatr Res 84, 411–418 (2018). https://doi.org/10.1038/s41390-018-0090-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-018-0090-0