Abstract
Objective
To determine changes in the intraamniotic environment during the latency period using paired amniotic and gastric fluid samples in pregnancies complicated by preterm prelabor rupture of membranes (PPROM).
Methods
A total of 34 women with singleton pregnancies complicated by PPROM prior to 34 weeks were included in the study. Amniotic fluid was obtained by transabdominal amniocentesis at the time of admission. Immediately after delivery, umbilical cord blood and gastric fluid were obtained.
Result
Microorganisms in amniotic and gastric fluid samples were found in 38% and 59% of women, respectively. Bedside IL-6 levels were higher in amniotic than in gastric fluid in pregnancies without fetal inflammatory response syndrome (FIRS) (263 pg/mL vs. 50 pg/mL; p < 0.0001), but not in pregnancies with FIRS (318 pg/mL vs. 444 pg/mL; p = 0.91). Funisitis and FIRS was associated with the highest bedside IL-6 levels in gastric fluid. A gastric fluid bedside IL-6 level of 275 pg/mL was found to be the ideal cutoff value to predict funisitis and FIRS.
Conclusions
The microbial and inflammatory status of the intraamniotic compartment changes during the latency period in PPROM. Bedside IL-6 assessment of gastric fluid may be useful in the rapid diagnosis of funisitis and FIRS.
Similar content being viewed by others
Introduction
Preterm prelabor rupture of membranes (PPROM) is defined as a rupture of fetal membranes with leakage of amniotic fluid before the onset of regular uterine activity prior to gestational age of 37 weeks.1 PPROM, which is responsible for about one-third of all preterm deliveries, still represents a serious clinical problem since the methods and techniques useful in the prediction and prevention of PPROM have yet to be revealed.1,2 PPROM at gestational age <34 weeks is responsible for about one-half of all cases, and is related to higher neonatal morbidity than in PPROM beyond 34 weeks.3,4,5,6
Pregnancies with PPROM, mainly before gestational age of 34 weeks, can be complicated by the presence of microorganisms in amniotic fluid and/or by elevated levels of inflammatory mediators in amniotic fluid.7,8 These conditions, known as microbial invasion of the amniotic cavity (MIAC) and intraamniotic inflammation (IAI), may lead to the development of acute inflammation of the fetal membranes, placenta, and umbilical cord, known as acute histological chorioamnionitis (HCA) and funisitis.9,10,11 These complications can result in fetal inflammatory response syndrome (FIRS), which is associated with worse neonatal short and long-term outcomes.12
Intraamniotic complications are typically diagnosed through the evaluation of an amniotic fluid sample obtained by transabdominal amniocentesis, mainly at the time of admission.13,14 Nevertheless, these complications, along with HCA, funisitis, and FIRS may develop subclinically during the latency period (the interval between PPROM and delivery), due to secondary infection caused by ascent of bacteria from the vagina, cervix, or the choriodecidual space to the intraamniotic compartment.8 Thus, these complications may represent a hidden threat for the fetus and newborn, even in pregnancies undergoing invasive sampling of amniotic fluid. Given that expectant management with a long latency period is the usual standard management approach for PPROM at gestational age <34 weeks, this subgroup of PPROM is the most jeopardized by secondary infection leading to HCA and FIRS.1
It is not usually possible to perform repeated amniocentesis or to obtain amniotic fluid directly at the time of delivery. However, as the swallowed amniotic fluid represents the main component of gastric fluid, aspiration from the newborn may serve as a surrogate of amniotic fluid to characterize the intraamniotic environment at the time of delivery.15,16 Therefore, changes in the gastric fluid composition may accurately reflect the presence of funisitis and FIRS.
Other than the pioneering works by Romero et al.8 and Gomez et al.17 showing an increase in MIAC, there is little information about changes in the inflammatory and infection-related status in the intraamniotic compartment during the latency period in PPROM.
Therefore, the primary aim was to characterize the changes in the intraamniotic environment during the latency period using paired amniotic and gastric fluid samples obtained at the time of admission and immediately after delivery, respectively, in pregnancies complicated by PPROM at <34 weeks. Secondary aims were to compare the diagnostic indices of amniotic and gastric fluids to predict funisitis and FIRS.
Materials and methods
Patient population
A prospective cohort study of pregnant women with PPROM between gestational ages 24 + 0 and 33 + 6 weeks, who were admitted to the department of Obstetrics and Gynecology, University Hospital Hradec Kralove between January 2016 and December 2016, was conducted. Women with singleton pregnancies who were at least 18 years old were included in the study. Women with diabetes mellitus, gestational diabetes mellitus, preeclampsia, chronic or pregnancy-induced hypertension, presence of chromosomal or structural fetal abnormalities, signs of fetal hypoxia, or significant vaginal bleeding were excluded from the study.
Gestational ages were established by first-trimester fetal biometry. Women were treated with antibiotics and corticosteroids to accelerate lung maturation. Those with the presence of both MIAC and IAI (bedside amniotic fluid interleukin (IL)-6 ≥ 745 pg/mL) beyond 28 gestational weeks were actively managed. In actively managed women, labor was induced, or an elective cesarean section was performed within 72 h of membrane rupture. The remaining women were managed conservatively. PPROM was diagnosed by examination with a sterile speculum to verify the pooling of amniotic fluid in the vagina. In cases of clinical uncertainty, amniotic fluid leakage was confirmed by the presence of insulin-like growth factor-binding protein (ACTIM PROM test; MedixBiochemica, Kauniainen, Finland) in the vaginal fluid.
Sample collection
Ultrasound-guided transabdominal amniocentesis was performed upon admission, before administration of corticosteroids and antibiotics; ~1–2 mL of amniotic fluid was obtained. A total of 100 μL of non-centrifuged amniotic fluid was used for bedside assessment of IL-6 levels. The remaining amniotic fluid was immediately transported to the microbiology laboratory for polymerase chain reaction (PCR) testing for Ureaplasma species, Mycoplasma hominis, and Chlamydia trachomatis, and for 16S rRNA gene, as well as for aerobic/anaerobic cultivation of amniotic fluid.
Umbilical blood samples were obtained by venipuncture from clamped umbilical cords immediately after delivery and prior to delivery of the placenta using a Vacutainer blood collecting system. The samples of umbilical cord blood were immediately centrifuged and aliquoted, and the supernatants were stored at −70 °C until assayed.
Gastric fluid was collected from the newborn within 10 min after delivery, when a sterile nasogastric tube was inserted. The samples of gastric fluid were immediately centrifuged and aliquoted. The pellets and supernatants were stored at −70 °C until assayed.
After delivery, the placenta, fetal membranes, and the umbilical cord were fixed in 10% neutral buffered formalin. Tissue samples were obtained from the placenta and fetal membranes (at least 2 samples), umbilical cord (usually 1 sample), and placental membranes (at least 2 samples) and routinely processed and embedded in paraffin. Sections of tissue blocks were stained with hematoxylin and eosin.
The study was approved by the institutional review board committee (July 2014; No 201407 S14P). All women provided written informed consent and were self-reported as Caucasians. Amniotic fluid from women in this cohort was used in some of our previous work.
Amniotic fluid and gastric bedside IL-6 levels
Fresh uncentrifuged samples of amniotic fluid and centrifuged samples of gastric fluid that underwent one freeze-thaw cycle were used for bedside IL-6 measurement. IL-6 levels were assessed with a lateral flow immunoassay, Milenia® QuickLine IL-6, using the MileniaPOCScan Reader (Millenia Biotec, GmbH, Giessen, Germany). The measurement range was 50–10,000 pg/mL. The intra-assay and inter-assay variations were 12.1% and 15.5%, respectively.
Umbilical cord blood IL-6 levels
Umbilical cord blood IL-6 levels were assessed using an enzyme-linked immunosorbent assay, Human IL-6 Quantikine (R&D Systems Inc., Minneapolis, MN). The sensitivity of the test was <0.70 pg/mL, and the inter-assay and intra-assay coefficients were <10%.
Detection of Ureaplasma species, Mycoplasma hominis, and Chlamydia trachomatis in amniotic and gastric fluids
DNA was isolated from fresh amniotic fluid and from pellets of gastric fluids with a QIAamp DNA Mini Kit (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions (using a protocol for bacterial DNA isolation from biological fluids). Real-time PCR was performed with a Rotor-Gene 6000 instrument (QIAGEN, Hilden, Germany), using a commercial AmpliSens® C. trachomatis/Ureaplasma/M. hominis-FRT kit (Federal State Institution of Science, Central Research Institute of Epidemiology, Moscow, Russia) to detect DNA from Ureaplasma species, M. hominis, and C. trachomatis in a common PCR tube. As a control, we included a PCR run for beta-actin, a housekeeping gene, to determine the presence of PCR inhibitors. The amount of Ureaplasma species DNA in copies/mL was determined by an absolute quantification technique employing an external calibration curve. Plasmid DNA (pCR4, Invitrogen) was used to prepare the calibration curve. Specific PCR for Ureaplasma species, M. hominis or C. trachomatis had a sensitivity of 50–100 copies/mL.
Non-cultivation detection of other bacteria in amniotic and gastric fluids
Bacterial DNA was identified using PCR targeting the 16S rRNA gene with the following primers: 5′-CCAGACTCCTACGGGAGGCAG-3′ (V3 region) and 5′-ACATTTCACAACACGAGCTGACGA-3′ (V6 region).18,19 Each individual reaction contained 3 μL of target DNA, 500 nM of forward and reverse primers, and Q5 High Fidelity DNA polymerase (NEB, Hitchin, UK) in a total volume of 25 μL. The amplification was performed in a 2720 Thermal Cycler (Applied Biosystems, Foster City, CA). The products were visualized on agarose gel. Positive reactions yielded products of 950 bp, which were subsequently analyzed by sequencing. The 16S PCR products were cleaned and used in sequencing PCR reactions utilizing the above primers and the BigDye Terminator kit, version 3.1 (Applied Biosystems, Foster City, CA). The bacteria were then typed using the sequences obtained in BLAST® and SepsiTestTM BLAST. The sensitivity of our method for detection of bacterial 16S rRNA, verified using DNA of Streptococcus agalactiae, S. mutans, and Haemophilus influenzae, was 150–700 copies/mL. Sanger sequencing of 16S rRNA was only possible when the concentration of bacterial DNA in amniotic fluid was at least 1500–7000 copies/mL.
Diagnosis of HCA and funisitis
The degree of polymorphonuclear leukocyte infiltration was assessed separately in the free membranes (amnion and chorion-decidua), in the chorionic plate, and in the umbilical cord, according to the criteria reported by Salafia et al.20 Diagnosis of HCA was made based on the presence of inflammatory changes in the chorion-decidua (grades 3–4), chorionic plate (grades 3–4), umbilical cord (grades 1–4), and/or amnion (grades 1–4). Diagnosis of funisitis was determined by the presence of inflammatory changes in the umbilical cord (grades 1–4).20
Diagnosis of FIRS
FIRS was defined by an umbilical cord blood IL-6 level >11.0 pg/mL.12
Statistical analysis
The demographic and clinical characteristics were compared using a nonparametric Mann–Whitney U test and presented as medians (interquartile range (IQR)) for continuous variables. Categorical variables were compared using Fisher’s exact test and were presented as numbers (%). The normality of the data was tested using the D’Agostino and Pearson omnibus normality test. Because the amniotic and gastric fluid IL-6 levels were not normally distributed, the nonparametric paired Wilcoxon test and the nonparametric unpaired Mann–Whitney U or Jonckheere–Terpstra tests were used for the analyses, as appropriate. The partial correlation was used to adjust the results for gestational age at sampling. The Spearman correlation was used to assess the association between the IL-6 levels in the gastric fluid and umbilical cord blood samples. Differences were considered statistically significant at p < 0.05. All p-values were from two-sided tests, and all statistical analyses were performed using SPSS 19.0 for Mac OS X (SPSS Inc., Chicago, IL) and with GraphPad Prism 6.0 h for Mac OS X (GraphPad Software, La Jolla, CA).
Results
Demographic and clinical characteristics of the study population
In total 37 women with PPROM at gestational ages 24 + 0 and 33 + 6 weeks were recruited during the study period. Three were excluded due to gestational diabetes mellitus (n = 1), chronic hypertension (n = 1), or duodenal atresia (n = 1). The remaining 34 women were included in the analysis. The maternal and neonatal characteristics of the study group are shown in Table 1.
Associations with the presence of microorganisms in amniotic and gastric fluids
No statistical difference was found in the presence of microorganisms between amniotic and gastric fluids (amniotic fluid: 38% (13/34) vs. gastric fluid: 59% (20/34); p = 0.14). No statistical difference was found in the presence of microorganisms in amniotic fluid between pregnancies with and without FIRS (54% (7/13) vs. 29% (6/21), respectively; p = 0.17); however, pregnancies with FIRS had a higher rate of the presence of microorganisms in the gastric fluid that those without FIRS (with 85% (11/13) vs. without 43% (9/21); p = 0.03).
Ureaplasma was the most common species identified in both amniotic fluid (n = 8) and gastric fluid (n = 14). No polymicrobial finding was identified in amniotic fluid, but two were found in gastric fluid (Ureaplasma species+M. hominis; Ureaplasma species+M. hominis+C. trachomatis). The microbial findings in the paired amniotic and gastric fluid samples are shown in Table 2. A positive correlation between microbial loads of Ureaplasma species and the bedside IL-6 levels was revealed in gastric fluid (rho = 0.67; p = 0.01). All 3 newborns who developed early-onset sepsis had positive microbial finding in gastric fluid (2 with Escherichia coli and 1 with S. agalactiae).
Associations with bedside IL-6 levels in amniotic and gastric fluids
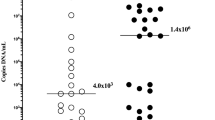
A difference was observed between bedside IL-6 levels in amniotic and gastric fluids (amniotic fluid: median 281 pg/mL, IQR 149–886 vs. gastric fluid: median 157 pg/mL, IQR 50–298; p = 0.02). When pregnancies were divided into subgroups based on FIRS, no difference in bedside IL-6 levels between amniotic and gastric fluids was identified in pregnancies with FIRS (amniotic fluid: median 318 pg/mL, IQR 157–10,000 vs. gastric fluid 444 pg/mL, IQR 230–10,000; p = 0.91; Fig. 1a). In pregnancies without FIRS, bedside IL-6 levels were higher in amniotic fluid than in gastric fluid (amniotic fluid: median 263 pg/mL, IQR 136–558 vs. gastric fluid: median 50 pg/mL, IQR 50–165; p < 0.0001; Fig. 1b).
Amniotic and gastric fluid IL-6 levels with respect to the presence of HCA and funisitis
No difference in amniotic fluid bedside IL-6 levels was found between pregnancies with and without funisitis or HCA (funisitis: median 181 pg/mL, IQR 72–7566; HCA without funisitis: median 583 pg/mL, IQR 360–5733; without HCA or funisitis: median 271 pg/mL, IQR 129–443; p = 0.96; Fig. 2a). However, differences were found in gastric fluid IL-6 levels (funisitis: median 772 pg/mL, IQR 250–10000; HCA without funisitis: median 155 pg/mL, IQR 54–198; without HCA or funisitis: median 50 pg/mL, IQR 50–158; p < 0.0001; Fig. 2b). The presence of funisitis was associated with higher bedside gastric fluid IL-6 levels than those found in the presence of HCA without funisitis (p = 0.006) and in the absence of HCA or funisitis (p = 0.0002) in crude analysis, as well as after adjustment for gestational age at delivery (p = 0.05, p = 0.03). No difference in gastric fluid bedside IL-6 levels between the presence HCA without funisitis and the absence of HCA or funisitis was identified (p = 0.18).
A gastric fluid IL-6 level of 275 pg/mL was found to be the ideal cutoff value for funisitis, with sensitivity of 75% (95% confidence interval (CI) 43–95; 9/12), specificity of 95% (95% CI 77–100; 21/22), positive predictive value of 90% (95% CI 56–100; 9/10), negative predictive value of 88% (95% CI 68–97; 21/24), odds ratio of 63, and area under the receiver operating characteristic curve of 88%.
Amniotic and gastric fluid IL-6 levels with respect to the presence of FIRS
No correlation was identified between amniotic fluid and umbilical cord blood IL-6 levels (rho = 0.31; p = 0.18; Fig. 3), but a positive correlation was found between gastric fluid and umbilical cord blood IL-6 levels (rho = 0.77; p < 0.0001; Fig. 3).
No difference in amniotic fluid bedside IL-6 levels was found between pregnancies with and without FIRS (with FIRS: median 318 pg/mL, IQR 157–10,000 vs. without FIRS: median 263 pg/mL, IQR 136–558; p = 0.36). However, pregnancies with FIRS had higher gastric fluid IL-6 levels than those without FIRS (with FIRS: median 444 pg/mL, IQR 230–10,000 vs. without FIRS: median 50, IQR 50–165; p < 0.0001; Fig. 4a) in crude analysis and after adjustment for gestational age at delivery (p = 0.0006).
A gastric fluid IL-6 level of 275 pg/mL was found to be the ideal cutoff value, with sensitivity of 77% (95% CI 46–95; 10/13), specificity of 100% (95% CI 84–100; 21/21), positive predictive value of 100% (95% CI 69–100; 10/10), negative predictive value of 88% (95% CI 68–97; 21/24), odds ratio of 129, and area under the receiver operating characteristic curve of 93% (Fig. 4b).
Comment
Principal findings of this study
First, no statistically significant differences were revealed between the rates (38% vs. 59%) of microbial presence in amniotic and gastric fluids obtained at the time of admission and immediately after delivery, respectively. Second, a positive correlation was found between microbial load with Ureaplasma species and bedside IL-6 levels in gastric fluid. Third, bedside IL-6 levels were only higher in amniotic than in gastric fluid in pregnancies without FIRS. Fourth, the presence of funisitis and FIRS was associated with the highest bedside IL-6 levels in gastric fluid. Last, a gastric fluid bedside IL-6 level of 275 pg/mL was found to be the ideal cutoff value to predict funisitis and FIRS.
Meaning of the study
Gastric fluid is usually obtained after delivery from preterm newborns by aspiration through an orogastric or nasogastric tube.21 The gastric fluid consists of amniotic, gastrointestinal, and lung components; however, the amniotic fluid represents a substantial constituent of the combined fluid.15,16,21,22 Therefore, it can be considered a surrogate of amniotic fluid at delivery.16
The presence of MIAC in PPROM at the time of admission varies between 20 and 50%, mainly based on gestational age and the methods used to identify bacteria.23,24 Information about the rate of MIAC at the time of delivery is sparse due to technical difficulties and ethical reasons preventing amniocentesis during delivery. Nevertheless, Romero et al.8 clearly showed that the rate of MIAC in PPROM increased during the latency period, and was as high as 75% at the time of delivery. In this study, we found an increase of about 20% during the latency period. However, this did not reach statistical significance (p = 0.14), likely owing to the small sample size. Interestingly, pregnancies with FIRS had a higher rate of microbial presence in gastric fluid than pregnancies without FIRS. This trend was not found in amniotic fluid. This suggests that secondary infection during the latency period is an important factor in the development of FIRS.
Ureaplasma species are the most common bacteria found in amniotic fluid from PPROM.25,26 Ureaplasma species are also the most common bacteria in gastric fluid.27 Similar to previous observations in amniotic fluid and maternal or umbilical cord blood, the intensity of inflammatory response in gastric fluid to Ureaplasma species was dose-dependent.28,29,30,31 This suggests that bedside IL-6 assessment of gastric fluid samples positive for Ureaplasma species might be useful in predicting the microbial burden.
The observation from paired samples used in a recent study showed a lower rate of Ureaplasma species in gastric fluid samples at the time of delivery than in amniotic fluid samples at the time of admission.27 This conflicts with the results in our study. We found a higher rate of Ureaplasma species in gastric fluid than in amniotic fluid. This is consistent with the observation that the rate of microbial presence in amniotic fluid increased during the latency period due to secondary infection.8 In addition, 2 women with a very low microbial load of Ureaplasma species in amniotic fluid (5000 and 7400 DNA copies/mL, respectively) did not have Ureaplasma species in gastric fluid but instead had E. coli. Newborns from these pregnancies and the newborn from the pregnancy in which S. agalactiae was found in both amniotic and gastric fluids developed early-onset sepsis. This observation is clinically relevant, suggesting that the presence of E. coli or S. agalactiae in gastric fluid might be related to a higher risk of early-onset sepsis. Therefore, the finding of these bacteria in gastric fluid should be of great concern and the newborns should be treated accordingly.
A recent study has shown that IL-6 levels in amniotic and gastric fluid obtained at the time of delivery are very similar.16 From a clinical point of view, this observation is relevant when the gastric fluid is considered as a surrogate of amniotic fluid to assess IL-6 levels. Given this fact, paired amniotic and gastric fluid samples obtained at different time points might be used to assess temporal changes in the inflammatory status of the intraamniotic compartment. This study found a generally decreasing trend in IL-6 levels as delivery approached, suggesting diminishing intraamniotic inflammation likely due to antibiotic treatment. When women were grouped by the development of FIRS, this trend was clearly seen in pregnancies that did not develop FIRS. On the other hand, pregnancies that developed FIRS did not demonstrate this clearly decreasing trend of IL-6 levels. In this group, both changes from high to low IL-6 levels as well as changes from low to high IL-6 levels were observed. This finding is clinically relevant, suggesting that FIRS might develop in PPROM at <34 weeks, irrespective of the IL-6 level at the time of admission.
Acute inflammatory changes in the placenta have traditionally been considered the histopathological counterparts of intraamniotic complications at the time of delivery.9,32 The ability to identify the presence of HCA and funisitis by evaluation of samples of amniotic fluid decreases with increasing time interval between amniotic fluid and placental sampling. Given the use of gastric fluid as a surrogate of amniotic fluid at delivery, it is not surprising that a recent publication showed an association of HCA and funisitis with gastric fluid IL-1β, IL-8, epithelial cell-derived neutrophil-activating peptide-78, and growth-related oncogene-alpha levels.21 In this study, we found that only funisitis was associated with higher gastric fluid IL-6 levels. As expected, gastric fluid had better predictive value for funisitis than amniotic fluid obtained at the time of admission.
The presence of FIRS, characterized by an IL-6 level in umbilical cord blood >11 pg/mL, is associated with changes in fetal organs leading to increased neonatal morbidity.12 FIRS represents an important threat for a preterm newborn. Therefore, a clinical suspicion of FIRS should be acted on as soon as possible. In this study, we found that bedside IL-6 assessment of gastric fluid is a strong predictor of FIRS, and is much better than bedside IL-6 evaluation of amniotic fluid obtained at the time of admission.
A gastric fluid bedside IL-6 level of 275 pg/mL was found to be the ideal cutoff for prediction of funisitis and FIRS. This finding may have clinical implications, since the bedside assessment of gastric fluid IL-6 can be performed directly at the location where the sample was obtained. In addition, it is a cost-effective, easy, and rapid test, with very good diagnostic performance.
The main strength of this study is the unique availability of a broad range of samples (from amniotic fluid, gastric fluid, umbilical cord blood, and placenta) from pregnancies complicated by PPROM at less than 34 weeks. Second, the characterization of microorganisms in amniotic and gastric fluids was based on non-cultivation approaches (non-specific PCR for 16S rRNA gene and specific PCR for Ureaplasma species, M. hominis, and C. trachomatis). This study had some limitations. First, the small group sample size increases the potential for a type II error. In addition, this sample size might be to small to give reasonable power. Therefore, the negative results should be taken with caution. Second, samples of amniotic fluid obtained at the time of delivery were lacking. Such samples would be ideal for confirmation of concordance between amniotic and gastric fluid at the time of delivery for the presence of microorganisms and bedside IL-6 levels. Third, the Sanger sequencing of 16S rRNA used in this study is only able to reveal the most common bacteria, but not all bacteria in a polymicrobial sample.
In conclusion, the microbial and inflammatory status of the intraamniotic compartment changes during the latency period in PPROM. The bedside IL-6 assessment of gastric fluid may be useful in the rapid diagnosis of funisitis and FIRS.
References
Mercer, B. M. Preterm premature rupture of the membranes. Obstet. Gynecol. 101, 178–193 (2003).
Kumar, D. et al. Progesterone inhibits in vitro fetal membrane weakening. Am. J. Obstet. Gynecol. 213, 520.e1–520.e9 (2015).
Musilova, I. et al. Intraamniotic inflammation in women with preterm prelabor rupture of membranes. PLoS ONE 10, e0133929 (2015).
van der Ham, D. P. et al. Induction of labor versus expectant management in women with preterm prelabor rupture of membranes between 34 and 37 weeks: a randomized controlled trial. PLoS Med. 9, e1001208 (2012).
Morris, J. M. et al. Immediate delivery compared with expectant management after preterm pre-labour rupture of the membranes close to term (PPROMT trial): a randomised controlled trial. Lancet 387, 444–452 (2016).
McElrath, T. F., Norwitz, E. R., Lieberman, E. S. & Heffner, L. J. Perinatal outcome after preterm premature rupture of membranes with in situ cervical cerclage. Am. J. Obstet. Gynecol. 187, 1147–1152 (2002).
Romero, R. et al. A comparative study of the diagnostic performance of amniotic fluid glucose, white blood cell count, interleukin-6, and gram stain in the detection of microbial invasion in patients with preterm premature rupture of membranes. Am. J. Obstet. Gynecol. 169, 839–851 (1993).
Romero, R. et al. Intraamniotic infection and the onset of labor in preterm premature rupture of the membranes. Am. J. Obstet. Gynecol. 159, 661–666 (1988).
Kim, C. J. et al. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. Am. J. Obstet. Gynecol. 213, S29–S52 (2015).
Oh, K. J. et al. Twenty-four percent of patients with clinical chorioamnionitis in preterm gestations have no evidence of either culture-proven intraamniotic infection or intraamniotic inflammation. Am. J. Obstet. Gynecol. 216, 604 e601–e604, e611 (2017).
Gomez-Lopez, N. et al. Are amniotic fluid neutrophils in women with intraamniotic infection and/or inflammation of fetal or maternal origin? Am. J. Obstet. Gynecol. 217, 693 e691–e693, e616 (2017).
Gomez, R. et al. The fetal inflammatory response syndrome. Am. J. Obstet. Gynecol. 179, 194–202 (1998).
Garite, T. J., Freeman, R. K., Linzey, E. M. & Braly, P. The use of amniocentesis in patients with premature rupture of membranes. Obstet. Gynecol. 54, 226–230 (1979).
Musilova, I. et al. Transabdominal amniocentesis is a feasible and safe procedure in preterm prelabor rupture of membranes. Fetal Diagn. Ther. 42, 257–261 (2017).
Brace, R. A. Physiology of amniotic fluid volume regulation. Clin. Obstet. Gynecol. 40, 280–289 (1979).
Kim, Y. D., Kim, S. C., Choi, K. U. & Jun, E. S. The relationship between amniotic and newborn gastric fluid inflammatory mediators. J. Matern. Fetal Neonatal Med. 26, 1069–1075 (2013).
Gomez, R. et al. Antibiotic administration to patients with preterm premature rupture of membranes does not eradicate intra-amniotic infection. J. Matern. Fetal Neonatal Med. 20, 167–173 (2007).
Fouhy, F. et al. The effects of freezing on faecal microbiota as determined using MiSeq sequencing and culture-based investigations. PLoS ONE 10, e0119355 (2015).
Greisen, K., Loeffelholz, M., Purohit, A. & Leong, D. PCR primers and probes for the 16S rRNA gene of most species of pathogenic bacteria, including bacteria found in cerebrospinal fluid. J. Clin. Microbiol. 32, 335–351 (1994).
Salafia, C. M., Weigl, C. & Silberman, L. The prevalence and distribution of acute placental inflammation in uncomplicated term pregnancies. Obstet. Gynecol. 73, 383–389 (1989).
Bry, K. J., Jacobsson, B., Nilsson, S. & Bry, K. Gastric fluid cytokines are associated with chorioamnionitis and white blood cell counts in preterm infants. Acta Paediatr. 104, 575–580 (2015).
Stichel, H. et al. Inflammatory cytokines in gastric fluid at birth and the development of bronchopulmonary dysplasia. Acta Paediatr. 100, 1206–1212 (2011).
DiGiulio, D. B. et al. Prevalence and diversity of microbes in the amniotic fluid, the fetal inflammatory response, and pregnancy outcome in women with preterm pre-labor rupture of membranes. Am. J. Reprod. Immunol. 64, 38–57 (2010).
Cobo, T. et al. Intra-amniotic inflammation predicts microbial invasion of the amniotic cavity but not spontaneous preterm delivery in preterm prelabor membrane rupture. Acta Obstet. Gynecol. Scand. 91, 930–935 (2012).
Yoon, B. H. et al. Microbial invasion of the amniotic cavity with Ureaplasma urealyticum is associated with a robust host response in fetal, amniotic, and maternal compartments. Am. J. Obstet. Gynecol. 179, 1254–1260 (1998).
Kacerovsky, M. et al. Amniotic fluid protein profiles of intraamniotic inflammatory response to Ureaplasma spp. and other bacteria. PLoS ONE 8, e60399 (2013).
Kim, S. M. et al. Gastric fluid versus amniotic fluid analysis for the identification of intra-amniotic infection due to Ureaplasma species. J. Matern. Fetal Neonatal Med. 29, 2579–2587 (2016).
Jacobsson, B., Aaltonen, R., Rantakokko-Jalava, K., Morken, N. H. & Alanen, A. Quantification of Ureaplasma urealyticum DNA in the amniotic fluid from patients in PTL and pPROM and its relation to inflammatory cytokine levels. Acta Obstet. Gynecol. Scand. 88, 63–70 (2009).
Kasper, D. C. et al. The bacterial load of Ureaplasma parvum in amniotic fluid is correlated with an increased intrauterine inflammatory response. Diagn. Microbiol. Infect. Dis. 67, 117–121 (2010).
Kacerovsky, M. et al. The microbial load with genital mycoplasmas correlates with the degree of histologic chorioamnionitis in preterm PROM. Am. J. Obstet. Gynecol. 205, 213.e1–213.e7 (2011).
Kacerovsky, M. et al. Microbial load of umbilical cord blood Ureaplasma species and Mycoplasma hominis in preterm prelabor rupture of membranes. J. Matern. Fetal Neonatal Med. 27, 1627–1632 (2014).
Salafia, C. M., Misra, D. & Miles, J. N. Methodologic issues in the study of the relationship between histologic indicators of intraamniotic infection and clinical outcomes. Placenta 30, 988–993 (2009).
Acknowledgements
This work was supported by the Faculty Hospital in Hradec Kralove (long-term organization development plan).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Musilova, I., Andrys, C., Hornychova, H. et al. Gastric fluid used to assess changes during the latency period in preterm prelabor rupture of membranes. Pediatr Res 84, 240–247 (2018). https://doi.org/10.1038/s41390-018-0073-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-018-0073-1