Abstract
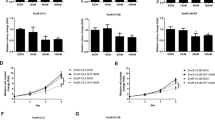
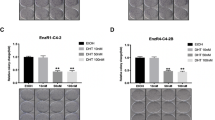
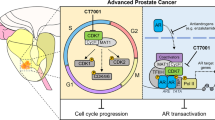
Castration-resistant prostate cancer (CRPC) is an aggressive disease with poor prognosis, and there is an urgent need for more effective therapeutic targets to address this challenge. Here, we showed that dihydroorotate dehydrogenase (DHODH), an enzyme crucial in the pyrimidine biosynthesis pathway, is a promising therapeutic target for CRPC. The transcript levels of DHODH were significantly elevated in prostate tumors and were negatively correlated with the prognosis of patients with prostate cancer. DHODH inhibition effectively suppressed CRPC progression by blocking cell cycle progression and inducing apoptosis. Notably, treatment with DHODH inhibitor BAY2402234 activated androgen biosynthesis signaling in CRPC cells. However, the combination treatment with BAY2402234 and abiraterone decreased intratumoral testosterone levels and induced apoptosis, which inhibited the growth of CWR22Rv1 xenograft tumors and patient-derived xenograft organoids. Taken together, these results establish DHODH as a key player in CRPC and as a potential therapeutic target for advanced prostate cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Data files and specific code used to perform all analyses in this manuscript are available at https://github.com/Wangjunjian-lab/DHODH-inhibition-improves-abiraterone-treatment-in-CRPC/tree/main.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Chen Y, Zhou Q, Hankey W, Fang X, Yuan F. Second generation androgen receptor antagonists and challenges in prostate cancer treatment. Cell Death Dis. 2022;13:632.
Mohler ML, Sikdar A, Ponnusamy S, Hwang DJ, He Y, Miller DD, et al. An overview of next-generation androgen receptor-targeted therapeutics in development for the treatment of prostate cancer. Int J Mol Sci. 2021;22:2124.
Posadas EM, Chi KN, de Wit R, de Jonge MJA, Attard G, Friedlander TW, et al. Pharmacokinetics, safety, and antitumor effect of apalutamide with abiraterone acetate plus prednisone in metastatic castration-resistant prostate cancer: phase Ib study. Clin Cancer Res. 2020;26:3517–24.
Davis ID, Martin AJ, Stockler MR, Begbie S, Chi KN, Chowdhury S, et al. Enzalutamide with standard first-line therapy in metastatic prostate cancer. New Engl J Med. 2019;381:121–31.
Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY, et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377:352–60.
Villa E, Ali ES, Sahu U, Ben-Sahra I. Cancer cells tune the signaling pathways to empower de novo synthesis of nucleotides. Cancers. 2019;11:688.
Löffler M, Fairbanks LD, Zameitat E, Marinaki AM, Simmonds HA. Pyrimidine pathways in health and disease. Trends Mol Med. 2005;11:430–7.
Evans DR, Guy HI. Mammalian pyrimidine biosynthesis: fresh insights into an ancient pathway. J Biol Chem. 2004;279:33035–8.
Scherer S, Oberle SG, Kanev K, Gerullis AK, Wu M, de Almeida GP, et al. Pyrimidine de novo synthesis inhibition selectively blocks effector but not memory T cell development. Nat Immunol. 2023;24:501–15.
Madak JT, Bankhead A 3rd, Cuthbertson CR, Showalter HD, Neamati N. Revisiting the role of dihydroorotate dehydrogenase as a therapeutic target for cancer. Pharmacol Ther. 2019;195:111–31.
Robinson AD, Eich ML, Varambally S. Dysregulation of de novo nucleotide biosynthetic pathway enzymes in cancer and targeting opportunities. Cancer Lett. 2020;470:134–40.
Hauser SL, Bar-Or A, Cohen JA, Comi G, Correale J, Coyle PK, et al. Ofatumumab versus teriflunomide in multiple sclerosis. N Engl J Med. 2020;383:546–57.
Zeng F, Li S, Yang G, Luo Y, Qi T, Liang Y, et al. Design, synthesis, molecular modeling, and biological evaluation of acrylamide derivatives as potent inhibitors of human dihydroorotate dehydrogenase for the treatment of rheumatoid arthritis. Acta Pharm Sin B. 2020;11:795–809.
Zhou J, Yiying Quah J, Ng Y, Chooi JY, Hui-Min Toh S, Lin B, et al. ASLAN003, a potent dihydroorotate dehydrogenase inhibitor for differentiation of acute myeloid leukemia. Haematologica. 2020;105:2286–97.
Zhou Y, Tao L, Zhou X, Zuo Z, Gong J, Liu X, et al. DHODH and cancer: promising prospects to be explored. Cancer Metab. 2021;9:22.
Mao C, Liu X, Zhang Y, Lei G, Yan Y, Lee H, et al. DHODH-mediated ferroptosis defence is a targetable vulnerability in cancer. Nature. 2021;593:586–90.
Pal S, Kaplan JP, Nguyen H, Stopka SA, Savani MR, Regan MS, et al. A druggable addiction to de novo pyrimidine biosynthesis in diffuse midline glioma. Cancer Cell. 2022;40:957–972.e910.
Gwynne WD, Suk Y, Custers S, Mikolajewicz N, Chan JK, Zador Z, et al. Cancer-selective metabolic vulnerabilities in MYC-amplified medulloblastoma. Cancer Cell. 2022;40:1488–1502.e1487.
Christian S, Merz C, Evans L, Gradl S, Seidel H, Friberg A, et al. The novel dihydroorotate dehydrogenase (DHODH) inhibitor BAY 2402234 triggers differentiation and is effective in the treatment of myeloid malignancies. Leukemia. 2019;33:2403–15.
Locke JA, Guns ES, Lubik AA, Adomat HH, Hendy SC, Wood CA, et al. Androgen levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancer. Cancer Res. 2008;68:6407–15.
Zhao J, Ning S, Lou W, Yang JC, Armstrong CM, Lombard AP, et al. Cross-resistance among next-generation antiandrogen drugs through the AKR1C3/AR-V7 axis in advanced prostate cancer. Mol Cancer Ther. 2020;19:1708–18.
Mitsiades N, Sung CC, Schultz N, Danila DC, He B, Eedunuri VK, et al. Distinct patterns of dysregulated expression of enzymes involved in androgen synthesis and metabolism in metastatic prostate cancer tumors. Cancer Res. 2012;72:6142–52.
Yang JC, Xu P, Ning S, Wasielewski LJ, Adomat H, Hwang SH, et al. Novel inhibition of AKR1C3 and androgen receptor axis by PTUPB synergizes enzalutamide treatment in advanced prostate cancer. Oncogene. 2023;42:693–707.
Mostaghel EA, Marck BT, Kolokythas O, Chew F, Yu EY, Schweizer MT, et al. Circulating and intratumoral adrenal androgens correlate with response to abiraterone in men with castration-resistant prostate cancer. Clin Cancer Res. 2021;27:6001–11.
Lorente D, Llacer C, Lozano R, de Velasco G, Romero-Laorden N, Rodrigo M, et al. Prognostic score and benefit from abiraterone in first-line metastatic, castration-resistant prostate cancer. Eur Urol. 2021;80:641–9.
Mei Z, Yang T, Liu Y, Gao Y, Hou Z, Zhuang Q, et al. Management of prostate cancer by targeting 3βHSD1 after enzalutamide and abiraterone treatment. Cell Rep Med. 2022;3:100608.
Swami U, McFarland TR, Nussenzveig R, Agarwal N. Advanced prostate cancer: treatment advances and future directions. Trends Cancer. 2020;6:702–15.
Teo MY, Rathkopf DE, Kantoff P. Treatment of advanced prostate cancer. Annu Rev Med. 2019;70:479–99.
Gillessen S, Armstrong A, Attard G, Beer TM, Beltran H, Bjartell A, et al. Management of patients with advanced prostate cancer: report from the advanced prostate cancer consensus conference 2021. Eur Urol. 2022;82:115–41.
Dellis AE, Papatsoris AG. Apalutamide: the established and emerging roles in the treatment of advanced prostate cancer. Expert Opin Investig Drugs. 2018;27:553–9.
Shore N, Zurth C, Fricke R, Gieschen H, Graudenz K, Koskinen M, et al. Evaluation of clinically relevant drug-drug interactions and population pharmacokinetics of darolutamide in patients with nonmetastatic castration-resistant prostate cancer: results of pre-specified and post hoc analyses of the phase III ARAMIS trial. Target Oncol. 2019;14:527–39.
Diehl FF, Miettinen TP, Elbashir R, Nabel CS, Darnell AM, Do BT, et al. Nucleotide imbalance decouples cell growth from cell proliferation. Nat Cell Biol. 2022;24:1252–64.
Yang C, Zhao Y, Wang L, Guo Z, Ma L, Yang R, et al. De novo pyrimidine biosynthetic complexes support cancer cell proliferation and ferroptosis defence. Nat Cell Biol. 2023;25:836–47.
Ma Y, Zhu Q, Wang X, Liu M, Chen Q, Jiang L, et al. Synthetic lethal screening identifies DHODH as a target for MEN1-mutated tumor cells. Cell Res. 2022;32:596–9.
Qian Y, Liang X, Kong P, Cheng Y, Cui H, Yan T, et al. Elevated DHODH expression promotes cell proliferation via stabilizing β-catenin in esophageal squamous cell carcinoma. Cell Death Dis. 2020;11:862.
Schweizer MT, Yu EY. Targeting intratumoral androgens: statins and beyond. Ther Adv Med Oncol. 2016;8:388–95.
Barnard M, Mostaghel EA, Auchus RJ, Storbeck KH. The role of adrenal derived androgens in castration resistant prostate cancer. J Steroid Biochem Mol Biol. 2020;197:105506.
Hettel D, Sharifi N. HSD3B1 status as a biomarker of androgen deprivation resistance and implications for prostate cancer. Nat Rev Urol. 2018;15:191–6.
Naelitz BD, Sharifi N. Through the looking-glass: reevaluating DHEA metabolism through HSD3B1 genetics. Trends Endocrinol Metab. 2020;31:680–90.
Ye L, Su ZJ, Ge RS. Inhibitors of testosterone biosynthetic and metabolic activation enzymes. Molecules. 2011;16:9983–10001.
Zhou J, Wang Y, Wu D, Wang S, Chen Z, Xiang S, et al. Orphan nuclear receptors as regulators of intratumoral androgen biosynthesis in castration-resistant prostate cancer. Oncogene. 2021;40:2625–34.
Stein MN, Goodin S, Dipaola RS. Abiraterone in prostate cancer: a new angle to an old problem. Clin Cancer Res. 2012;18:1848–54.
Huang JL, Yan XL, Li W, Fan RZ, Li S, Chen J, et al. Discovery of highly potent daphnane diterpenoids uncovers importin-β1 as a druggable vulnerability in castration-resistant prostate cancer. J Am Chem Soc. 2022;144:17522–32.
Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10:R25.
Li H, Durbin R. Fast and accurate long-read alignment with Burrows–Wheeler transform. Bioinformatics. 2010;26:589–95.
Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc. 2012;7:562–78.
Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, et al. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol. 2010;28:511–5.
Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–50.
Ross-Adams H, Lamb AD, Dunning MJ, Halim S, Lindberg J, Massie CM, et al. Integration of copy number and transcriptomics provides risk stratification in prostate cancer: a discovery and validation cohort study. EBioMedicine. 2015;2:1133–44.
Asangani IA, Dommeti VL, Wang X, Malik R, Cieslik M, Yang R, et al. Therapeutic targeting of BET bromodomain proteins in castration-resistant prostate cancer. Nature. 2014;510:278–82.
Nguyen HG, Yang JC, Kung HJ, Shi XB, Tilki D, Lara PN Jr, et al. Targeting autophagy overcomes enzalutamide resistance in castration-resistant prostate cancer cells and improves therapeutic response in a xenograft model. Oncogene. 2014;33:4521–30.
Acknowledgements
We thank the Laboratory Animal Center of Sun Yat-sen University for providing the technologies equipment.
Funding
This research was supported by the National Natural Science Foundation of China (82273956), the Guangdong Basic and Applied Basic Research Foundation (2022B1515130008), the Key Research and Development Plan of Guangzhou City (202206080007), and Science and Technology Planning Project of Guangdong Province (2023A0505010013).
Author information
Authors and Affiliations
Contributions
JJW, CFL, and GPZ conceived of and designed the experiments. SQG, MMM, YFW, and QYW performed the experiments and collected data. DYP and ZFK performed the bioinformatics analysis. SQG, JWZ, JJW, and CFL wrote and edited the manuscript. All authors agree with the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, S., Miao, M., Wu, Y. et al. DHODH inhibition represents a therapeutic strategy and improves abiraterone treatment in castration-resistant prostate cancer. Oncogene (2024). https://doi.org/10.1038/s41388-024-03005-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41388-024-03005-4