Abstract
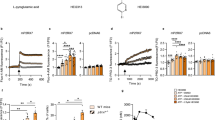
The effectiveness and mechanisms of γδT-cell immunotherapy in lung cancer remain unclear. In this study, we assessed the effects of continuous, low-dose γδT-cell intervention on lung cancer cells. We cultured γδT cells with a lung cancer cell line (A549) and replaced the γδT-cell population every 48 hours. The killing effect of γδTcells on A549 cells and the Half-maximal inhibitory concentration (IC50) value were detected by the cholecystokinin octapeptide (CCK-8) method. The levels of perforin, granzyme B and the inflammatory factors interleukin-6 (IL-6), interferon (IFN)-γ, and tumor necrosis factor-alpha (TNF-a), in the supernatants of cocultured cells were measured by ELISA. The protein expression of Bcl-2, Bax, PI3K and Akt was detected by western blotting. Our results indicated that γδT-cell treatment decreased the protein expression of Bcl-2, PI3K, and AKT but upregulated that of Bax. Moreover, γδT-cell treatment increased perforin and granzyme B release related to the Bax/Bcl-2 signaling pathway. In addition, γδT-cell-mediated cytolysis for A549 cells involved the PI3K/AKT pathway. In vivo results were consistent with the in vitro results. γδT-cell immunotherapy integrated regulation of a signaling pathway network involving the mutual regulation of apoptosis and proliferation. γδT-cell immunotherapy could be used to enhance the cytotoxic killing of lung cancer cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Howlader N, Forjaz G, Mooradian MJ, Meza R, Kong CY, Cronin KA, et al. The Effect of Advances in Lung-Cancer Treatment on Population Mortality. N Engl J Med. 2020;383:640–9.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Mamdani H, Matosevic S, Khalid AB, Durm G, Jalal SI. Immunotherapy in Lung Cancer: Current Landscape and Future Directions. Front Immunol. 2022;13:823618.
Alnaggar M, Xu Y, Li J, He J, Chen J, Li M, et al. Allogenic V9V2 T cell as new potential immunotherapy drug for solid tumor: a case study for cholangiocarcinoma. J Immunother Cancer. 2019;7:36.
Lo Presti E, Pizzolato G, Gulotta E, Cocorullo G, Gulotta G, Dieli F, et al. Current advances in gammadelta T cell-based tumor immunotherapy. Front Immunol. 2017;8:1401.
Silva-Santos B, Mensurado S, Coffelt SB. gammadelta T cells: pleiotropic immune effectors with therapeutic potential in cancer. Nat Rev Cancer. 2019;19:392–404.
Sebestyen Z, Prinz I, Déchanet-Merville J, Silva-Santos B, Kuball J. Translating gammadelta (gammadelta) T cells and their receptors into cancer cell therapies. Nat Rev Drug Discov. 2020;19:169–84.
Kabelitz D, Serrano R, Kouakanou L, Peters C, Kalyan S. Cancer immunotherapy with γδT cells: many paths ahead of us. Cell Mol Immunol. 2020;17:925–39.
Baugh R, Khalique H, Seymour LW. Convergent Evolution by Cancer and Viruses in Evading the NKG2D Immune Response. Cancers. 2020;12:3827.
Lai AY, Patel A, Brewer F, Evans K, Johannes K, González LE, et al. Cutting Edge: Bispecific γδT Cell Engager Containing Heterodimeric BTN2A1 and BTN3A1 Promotes Targeted Activation of Vγ9Vδ2+ T Cells in the Presence of Costimulation by CD28 or NKG2D. J Immunol. 2022;209:1475–80.
Aoki T, Matsushita H, Hoshikawa M, Hasegawa K, Kokudo N, Kakimi K. Adjuvant combination therapy with gemcitabine and autologous gammadelta T-cell transfer in patients with curatively resected pancreatic cancer. Cytotherapy. 2017;19:473–85.
Xu Y, Xiang Z, Alnaggar M, Kouakanou L, Li J, He J, et al. Allogeneic Vγ9Vδ2 T-cell immunotherapy exhibits promising clinical safety and prolongs the survival of patients with late-stage lung or liver cancer. Cell Mol Immunol. 2021;18:427–39.
Morris EC, Neelapu SS, Giavridis T, Sadelain M. Cytokine release syndrome and associated neurotoxicity in cancer immunotherapy. Nat Rev Immunol. 2022;22:85–96.
Wang H, Chen H, Liu S, Zhang J, Lu H, Somasundaram R, et al. Costimulation of γδTCR and TLR7/8 promotes Vδ2 T-cell antitumor activity by modulating mTOR pathway and APC function. J Immunother Cancer. 2021;9:e003339.
Zhang Z, Yang C, Li L, Zhu Y, Su K, Zhai L, et al. “γδT Cell-IL17A-Neutrophil” Axis Drives Immunosuppression and Confers Breast Cancer Resistance to High-Dose Anti-VEGFR2 Therapy. Front Immunol. 2021;12:699478.
Xie H, Yao J, Wang Y, Ni B. Exosome-transmitted circVMP1 facilitates the progression and cisplatin resistance of non-small cell lung cancer by targeting miR-524-5p-METTL3/SOX2 axis. Drug Deliv. 2022;29:1257–71.
Lu H, Shi T, Wang M, Li X, Gu Y, Zhang X, et al. B7-H3 inhibits the IFN-γ-dependent cytotoxicity of Vγ9Vδ2 T cells against colon cancer cells. Oncoimmunology. 2020;9:1748991.
Chen SJ, Wang SC, Chen YC. The Immunotherapy for Colorectal Cancer, Lung Cancer and Pancreatic Cancer. Int J Mol Sci. 2021;22:12836.
Song Y, Liu Y, Teo HY, Liu H. Targeting Cytokine Signals to Enhance γδT Cell-Based Cancer Immunotherapy. Front Immunol. 2022;13:914839.
Barisa M, Fowler D, Fisher J. Interplay between T-Cell Metabolism and Tumour Microenvironment Offers Opportunities for Therapeutic Intervention. Immunometabolism. 2021;3:210026.
Kroschinsky F, Stolzel F, von Bonin S, Beutel G, Kochanek M, Kiehl M, et al. New drugs, new toxicities: severe side effects of modern targeted and immunotherapy of cancer and their management. Crit Care. 2017;21:89.
Gong WJ, Qiu Y, Li MH, Chen LY, Li YY, Yu JQ, et al. Investigation of the risk factors to predict cytokine release syndrome in relapsed or refractory B-cell acute lymphoblastic leukemia patients receiving IL-6 knocking down anti-CD19 chimeric antigen receptor T-cell therapy. Front Immunol. 2022;13:922212.
Sun Y, Hu B, Stanley G, Harris ZM, Gautam S, Homer R, et al. IFN-γ Is Protective in Cytokine Release Syndrome-associated Extrapulmonary Acute Lung Injury. Am J Respir Cell Mol Biol. 2023;68:75–89.
Kim JS, Lee JY, Yang JW, Lee KH, Effenberger M, Szpirt W, et al. Immunopathogenesis and treatment of cytokine storm in COVID-19. Theranostics. 2021;11:316–29.
Velotti F, Barchetta I, Cimini FA, Cavallo MG. Granzyme B in Inflammatory Diseases: Apoptosis, Inflammation, Extracellular Matrix Remodeling, Epithelial-to-Mesenchymal Transition and Fibrosis. Front Immunol. 2020;11:587581.
Hoxhaj G, Manning BD. The PI3K-AKT network at the interface of oncogenic signalling and cancer metabolism. Nat Rev Cancer. 2020;20:74–88.
Park JH, Pyun WY, Park HW. Cancer Metabolism: Phenotype, Signaling and Therapeutic Targets. Cells. 2020;9:2308.
Li X, Lu H, Gu Y, Zhang X, Zhang G, Shi T, et al. Tim-3 suppresses the killing effect of Vγ9Vδ2 T cells on colon cancer cells by reducing perforin and granzyme B expression. Exp Cell Res. 2020;386:111719.
Acknowledgements
This study is supported by The Key Research and Development Program of Guangxi (No. GUIKEAB1850004) and the Science and Technology Development Plan Project of Jilin Province (No. 20210204157YY).
Author information
Authors and Affiliations
Contributions
TZ and JD conceived and designed the study and drafted the manuscript. ZC and QH collected, analyzed and interpreted the experimental data. YL and CY revised the manuscript for important intellectual content. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of the Affiliated Hospital of Youjiang Medical University for Nationalities.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, Y., Zhang, T., Deng, J. et al. The cytotoxicity of γδT cells in non-small cell lung cancer mediated via coordination of the BCL-2 and AKT pathways. Oncogene 42, 3648–3654 (2023). https://doi.org/10.1038/s41388-023-02852-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-023-02852-x