Abstract
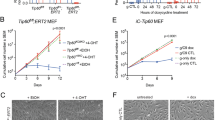
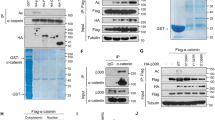
Williams syndrome transcription factor (WSTF) is a transcription factor and tyrosine kinase. WSTF overexpression promotes migration and proliferation of various cancers, and Ser158 (WSTFS158) phosphorylation plays an important role in this process. However, the role of the other posttranslational modifications of WSTF is unknown. Here, we report that lysine (K) 426 on WSTF is acetylated by MOF and deacetylated by SIRT1. Mechanistically, male-specific lethal (MSL) 1v1 interaction with WSTF facilitates its interaction with MOF for WSTF acetylation, which in turn promotes WSTFS158 phosphorylation. The kinase and transcriptional regulatory activity of WSTF were enhanced by acetylation. WSTFK426ac levels positively and significantly correlated with tumor size, histological grade, and age. Moreover, we demonstrated that acetylated WSTF promotes cancer cell proliferation, migration, invasion, and tumor formation. In conclusion, we identified the enzymes regulating WSTF K426 acetylation, and demonstrated an acetylation-dependent mechanism that modulates the activities of WSTF and contributes to tumorigenesis. Our findings provide new clues to study WSTF-mediated normal development and disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Barnett C, Krebs JE. WSTF does it all: a multifunctional protein in transcription, repair, and replication. Biochem Cell Biol. 2011;89:12–23.
Xiao A, Li H, Shechter D, Ahn SH, Fabrizio LA, Erdjument-Bromage H, et al. WSTF regulates the H2A.X DNA damage response via a novel tyrosine kinase activity. Nature. 2009;457:57–62.
Liu Y, Wang SQ, Long YH, Chen S, Li YF, Zhang JH. KRASG12 mutant induces the release of the WSTF/NRG3 complex, and contributes to an oncogenic paracrine signaling pathway. Oncotarget. 2016;7:53153–64.
Lundqvist J, Kirkegaard T, Laenkholm AV, Duun-Henriksen AK, Bak M, Feldman D, et al. Williams syndrome transcription factor (WSTF) acts as an activator of estrogen receptor signaling in breast cancer cells and the effect can be abrogated by 1alpha,25-dihydroxyvitamin D3. J Steroid Biochem Mol Biol. 2018;177:171–8.
Meng J, Zhang XT, Liu XL, Fan L, Li C, Sun Y, et al. WSTF promotes proliferation and invasion of lung cancer cells by inducing EMT via PI3K/Akt and IL-6/STAT3 signaling pathways. Cell Signal. 2016;28:1673–82.
Wang YQ, Liu Y, Li YF, Zhang JH, Liu YK, Li YH, et al. Williams syndrome transcription factor is a target of pro-oncogenic Ser158 phosphorylation mediated by Ras-MAPK pathway in human breast cancer. Int J Clin Exp Pathol. 2016;9:1668–75.
Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC, et al. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325:834–40.
Kouzarides T. Acetylation: a regulatory modification to rival phosphorylation? EMBO J. 2000;19:1176–9.
Kahali B, Gramling SJ, Marquez SB, Thompson K, Lu L, Reisman D. Identifying targets for the restoration and reactivation of BRM. Oncogene. 2014;33:653–64.
Yang XJ. The diverse superfamily of lysine acetyltransferases and their roles in leukemia and other diseases. Nucleic Acids Res. 2004;32:959–76.
Li X, Wu L, Corsa CA, Kunkel S, Dou Y. Two mammalian MOF complexes regulate transcription activation by distinct mechanisms. Mol Cell. 2009;36:290–301.
Su J, Wang F, Cai Y, Jin J The Functional Analysis of Histone Acetyltransferase MOF in Tumorigenesis. Int J Mol Sci. 2016;17:99. https://doi.org/10.3390/ijms17010099.
Pfister S, Rea S, Taipale M, Mendrzyk F, Straub B, Ittrich C, et al. The histone acetyltransferase hMOF is frequently downregulated in primary breast carcinoma and medulloblastoma and constitutes a biomarker for clinical outcome in medulloblastoma. Int J Cancer. 2008;122:1207–13.
Liu Y, Wang DL, Chen S, Zhao L, Sun FL. Oncogene Ras/phosphatidylinositol 3-kinase signaling targets histone H3 acetylation at lysine 56. J Biol Chem. 2012;287:41469–80.
Wan J, Xu W, Zhan J, Ma J, Li X, Xie Y, et al. PCAF-mediated acetylation of transcriptional factor HOXB9 suppresses lung adenocarcinoma progression by targeting oncogenic protein JMJD6. Nucleic Acids Res. 2016;44:10662–75.
Cook PJ, Ju BG, Telese F, Wang X, Glass CK, Rosenfeld MG. Tyrosine dephosphorylation of H2AX modulates apoptosis and survival decisions. Nature. 2009;458:591–6.
Liu Y, Long YH, Wang SQ, Zhang YY, Li YF, Mi JS, et al. JMJD6 regulates histone H2A.X phosphorylation and promotes autophagy in triple-negative breast cancer cells via a novel tyrosine kinase activity. Oncogene. 2019;38:980–97.
Das C, Lucia MS, Hansen KC, Tyler JK. CBP/p300-mediated acetylation of histone H3 on lysine 56. Nature. 2009;459:113–7.
Liu Y, Xing ZB, Wang SQ, Chen S, Liu YK, Li YH, et al. MDM2-MOF-H4K16ac axis contributes to tumorigenesis induced by Notch. FEBS J. 2014;281:3315–24.
Liu Y, Xing ZB, Zhang JH, Fang Y. Akt kinase targets the association of CBP with histone H3 to regulate the acetylation of lysine K18. FEBS Lett. 2013;587:847–53.
Guo J, Dai X, Laurent B, Zheng N, Gan W, Zhang J, et al. AKT methylation by SETDB1 promotes AKT kinase activity and oncogenic functions. Nat Cell Biol. 2019;21:226–37.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant number 81772834); Natural Science Foundation of Hebei Province (grant number H2019105034); Science Fund for Distinguished Young Scholars of North China University of Science and Technology (JQ201704); Doctoral Research Project of North China University of Science and Technology; the Program for Innovation Research Team (in Science and Technology) of Tangshan (17130205D).
Author information
Authors and Affiliations
Contributions
XJZ and JHZ had the idea for the experiment and supervised the project. YL, YYZ, YHL, SQW, ML, YQW and YFL, YKL, YHL, JSM, CHY, and DYL carried out the experiment. YL, SQW, and YHL wrote the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, Y., Zhang, YY., Wang, SQ. et al. WSTF acetylation by MOF promotes WSTF activities and oncogenic functions. Oncogene 39, 5056–5067 (2020). https://doi.org/10.1038/s41388-020-1350-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-020-1350-0
This article is cited by
-
The emerging role of ISWI chromatin remodeling complexes in cancer
Journal of Experimental & Clinical Cancer Research (2021)