Abstract
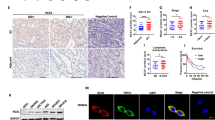
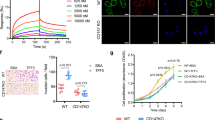
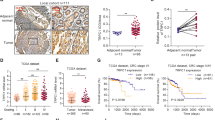
Although VPAC1 and its ligand vasoactive intestinal peptide (VIP) are important in gastrointestinal physiology, their involvements in progression of gastrointestinal tumor have not been explored. Here, we found that higher expression of VIP/VPAC1 was observed in gastric cancer compared to the adjacent normal tissues. The increased expression of VIP/VPAC1 in gastric cancer correlated positively with invasion, tumor stage, lymph node, distant metastases, and poor survival. Moreover, high expression of VIP and VPAC1, advanced tumor stage and distant metastasis were independent prognostic factors. VPAC1 activation by VIP markedly induced TRPV4-mediated Ca2+ entry, and eventually promoted gastric cancer progression in a Ca2+ signaling-dependent manner. Inhibition of VPAC1 and its signaling pathway could block the progressive responses. VPAC1/TRPV4/Ca2+ signaling in turn enhanced the expression and secretion of VIP in gastric cancer cells, enforcing a positive feedback regulation mechanism. Taken together, our study demonstrate that VPAC1 is significantly overexpressed in gastric cancer and VPAC1/TRPV4/Ca2+ signaling axis could enforce a positive feedback regulation in gastric cancer progression. VIP/VPAC1 may serve as potential prognostic markers and therapeutic targets for gastric cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. Cancer J Clin. 2016;66:7–30.
Tan P, Yeoh KG. Genetics and molecular pathogenesis of gastric adenocarcinoma. Gastroenterology. 2015;149:1153–62.
Badgwell B, Blum M, Estrella J, Ajani J. Personalised therapy for localised gastric and gastro-oesophageal adenocarcinoma. Lancet Oncol. 2016;17:1628–9.
Vacas E, Bajo AM, Schally AV, Sánchez-Chapado M, Prieto JC, Carmena MJ. Vasoactive intestinal peptide induces oxidative stress and suppresses metastatic potential in human clear cell renal cell carcinoma. Mol Cell Endocrinol. 2013;365:212–22.
Vacas E, Arenas MI, Muñoz-Moreno L, Bajo AM, Sánchez-Chapado M, Prieto JC, et al. Antitumoral effects of vasoactive intestinal peptide in human renal cell carcinoma xenografts in athymic nude mice. Cancer Lett. 2013;336:196–203.
Seoane IV, Ortiz AM, Piris L, Lamana A, Juarranz Y, García-Vicuña R, et al. Clinical relevance of VPAC1 receptor expression in early arthritis: association with IL-6 and disease activity. PLoS One. 2016;11:e0149141.
White CM, Ji S, Cai H, Maudsley S, Martin B. Therapeutic potential of vasoactive intestinal peptide and its receptors in neurological disorders. CNS Neurol Disord Drug Targets. 2010;9:661–6.
Valdehita A, Bajo AM, Schally AV, Varga JL, Carmena MJ, Prieto JC. Vasoactive intestinal peptide (VIP) induces transactivation of EGFR and HER2 in human breast cancer cells. Mol Cell Endocrinol. 2009;302:41–8.
Valdehita A, Carmena MJ, Collado B, Prieto JC, Bajo AM. Vasoactive intestinal peptide (VIP) increases vascular endothelial growth factor (VEGF) expression and secretion in human breast cancer cells. Regul Pept. 2007;144:101–8.
Reubi JC, Läderach U, Waser B, Gebbers JO, Robberecht P, Laissue JA. Vasoactive intestinal peptide/pituitary adenylate cyclase-activating peptide receptor subtypes in human tumors and their tissues of origin. Cancer Res. 2000;60:3105–12.
Fernández-Martínez AB, Carmena MJ, Arenas MI, Bajo AM, Prieto JC, Sánchez-Chapado M. Overexpression of vasoactive intestinal peptide receptors and cyclooxygenase-2 in human prostate cancer. Anal Potential Progn Relev Histol Histopathol. 2012;27:1093–101.
Liu S, Zeng Y, Li Y, Guo W, Liu J, Ouyang N. VPAC1 overexpression is associated with poor differentiation in colon cancer. Tumour Biol. 2014;35:6397–404.
Dickson L, Finlayson K. VPAC and PAC receptors: from ligands to function. Pharmacol Ther. 2009;121:294–316.
Zhang S, Liu Y, Guo S, Zhang J, Chu X, Jiang C, et al. Vasoactive intestinal polypeptide relaxes isolated rat pulmonary artery rings through two distinct mechanisms. J Physiol Sci. 2010;60:389–97.
Herrera JL, Gonzalez-Rey E, Fernandez-Montesinos R, Quintana FJ, Najmanovich R, Pozo D. Toll-like receptor stimulation differentially regulates vasoactive intestinal peptide type 2 receptor in macrophages. J Cell Mol Med. 2009;13:3209–17.
Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–29.
Monteith GR, Davis FM, Roberts-Thomson SJ. Calcium channels and pumps in cancer: changes and consequences. J Biol Chem. 2012;287:31666–73.
Fiorio Pla A, Ong HL, Cheng KT, Brossa A, Bussolati B, Lockwich T, et al. TRPV4 mediates tumor-derived endothelial cell migration via arachidonic acid-activated actin remodeling. Oncogene. 2012;31:200–12.
Wei C, Wang X, Chen M, Ouyang K, Song LS, Cheng H. Calcium flickers steer cell migration. Nature. 2009;457:901–5.
Evans AM, Fameli N, Ogunbayo OA, Duan J, Navarro-Dorado J. From contraction to gene expression: nanojunctions of the sarco/endoplasmic reticulum deliver site- and function-specific calcium signals. Sci China Life Sci. 2016;59:749–63.
Hofer AM, Brown EM. Extracellular calcium sensing and signalling. Nat Rev Mol Cell Biol. 2003;4:530–8.
Déliot N, Constantin B. Plasma membrane calcium channels in cancer: alterations and consequences for cell proliferation and migration. Biochim Biophys Acta. 2015;1848:2512–22.
Holzer P. TRP channels in the digestive system. Curr Pharm Biotechnol. 2011;12:24–34.
D’Aldebert E, Cenac N, Rousset P, Martin L, Rolland C, Chapman K, et al. Transient receptor potential vanilloid 4 activated inflammatory signals by intestinal epithelial cells and colitis in mice. Gastroenterology. 2011;140:275–85.
Xie R, Xu J, Xiao Y, Wu J, Wan H, Tang B, et al. Calcium promotes human gastric cancer via a novel coupling of calcium-sensing receptor and TRPV4 channel. Cancer Res. 2017;77:6499–512.
Chen YF, Hsu KF, Shen MR. The store-operated Ca(2+) entry-mediated signaling is important for cancer spread. Biochim Biophys Acta. 2016;1863:1427–35.
Chen YF, Chen YT, Chiu WT, Shen MR. Remodeling of calcium signaling in tumor progression. J Biomed Sci. 2013;20:23.
Zhu MX, Tuo B, Yang JJ. The hills and valleys of calcium signaling. Sci China Life Sci. 2016;59:743–8.
Leuner K, Heiser JH, Derksen S, Mladenov MI, Fehske CJ, Schubert R, et al. Simple 2,4-diacylphloroglucinols as classic transient receptor potential-6 activators—identification of a novel pharmacophore. Mol Pharmacol. 2010;77:368–77.
Storch U, Forst AL, Pardatscher F, Erdogmus S, Philipp M, Gregoritza M, et al. Dynamic NHERF interaction with TRPC4/5 proteins is required for channel gating by diacylglycerol. Proc Natl Acad Sci USA. 2017;114:E37–E46.
Xiao G, Wang X, Wang J, Zu L, Cheng G, Hao M, et al. CXCL16/CXCR6 chemokine signaling mediates breast cancer progression by pERK1/2-dependent mechanisms. Oncotarget. 2015;6:14165–78.
Song W, Ma Y, Wang J, Brantley-Sieders D, Chen J. JNK signaling mediates EPHA2-dependent tumor cell proliferation, motility, and cancer stem cell-like properties in non-small cell lung cancer. Cancer Res. 2014;74:2444–54.
Ma Y, Yang Y, Wang F, Moyer MP, Wei Q, Zhang P, et al. Long non-coding RNA CCAL regulates colorectal cancer progression by activating Wnt/β-catenin signalling pathway via suppression of activator protein 2α. Gut. 2016;65:1494–504.
Hejna M, Hamilton G, Brodowicz T, Haberl I, Fiebiger WC, Scheithauer W, et al. Serum levels of vasoactive intestinal peptide (VIP) in patients with adenocarcinomas of the gastrointestinal tract. Anticancer Res. 2001;21:1183–7.
Yin Y, Gao D, Wang Y, Wang ZH, Wang X, Ye J, et al. Tau accumulation induces synaptic impairment and memory deficit by calcineurin-mediated inactivation of nuclear CaMKIV/CREB signaling. Proc Natl Acad Sci USA. 2016;113:E3773–3781.
Hogan PG. Calcium-NFAT transcriptional signalling in T cell activation and T cell exhaustion. Cell Calcium. 2017;63:66–9.
Chen F, Zhuang X, Lin L, Yu P, Wang Y, Shi Y, et al. New horizons in tumor microenvironment biology: challenges and opportunities. BMC Med. 2015;13:45.
Galione A, Churchill GC. Interactions between calcium release pathways: multiple messengers and multiple stores. Cell Calcium. 2002;32:343–54.
Li D, Jiao J, Shatos MA, Hodges RR, Dartt DA. Effect of VIP on intracellular [Ca2+], extracellular regulated kinase 1/2, and secretion in cultured rat conjunctival goblet cells. Invest Ophthalmol Vis Sci. 2013;54:2872–84.
Hagen BM, Bayguinov O, Sanders KM. VIP and PACAP regulate localized Ca2+ transients via cAMP-dependent mechanism. Am J Physiol Cell Physiol. 2006;291:C375–85.
Woo DH, Jung SJ, Zhu MH, Park CK, Kim YH, Oh SB, et al. Direct activation of transient receptor potential vanilloid 1(TRPV1) by diacylglycerol (DAG). Mol Pain. 2008;4:42.
Kodama D, Togari A. Store-operated calcium entry induced by activation of Gq-coupled alpha1B adrenergic receptor in human osteoblast. Biochem Biophys Res Commun. 2013;437:239–44.
Andrikopoulos P, Kieswich J, Harwood SM, Baba A, Matsuda T, Barbeau O, et al. Endothelial angiogenesis and barrier function in response to thrombin require Ca2+ Influx through the Na+/Ca2+ exchanger. J Biol Chem. 2015;290:18412–28.
Favia A, Desideri M, Gambara G, D’Alessio A, Ruas M, Esposito B, et al. VEGF-induced neoangiogenesis is mediated by NAADP and two-pore channel-2-dependent Ca2+ signaling. Proc Natl Acad Sci USA. 2014;111:E4706–4715.
Thrasivoulou C, Millar M, Ahmed A. Activation of intracellular calcium by multiple Wnt ligands and translocation of β-catenin into the nucleus: a convergent model of Wnt/Ca2+ and Wnt/β-catenin pathways. J Biol Chem. 2013;288:35651–9.
Tabuchi A, Sakaya H, Kisukeda T, Fushiki H, Tsuda M. Involvement of an upstream stimulatory factor as well as cAMP-responsive element-binding protein in the activation of brain-derived neurotrophic factor gene promoter I. J Biol Chem. 2002;277:35920–31.
Hahm SH, Eiden LE. Cis-regulatory elements controlling basal and inducible VIP gene transcription. Ann N Y Acad Sci. 1998;865:10–26.
Schwarz EC, Qu B, Hoth M. Calcium, cancer and killing: the role of calcium in killing cancer cells by cytotoxic T lymphocytes and natural killer cells. Biochim Biophys Acta. 2013;1833:1603–11.
Tompkins JD, Girard BM, Vizzard MA, Parsons RL. VIP and PACAP effects on mouse major pelvic ganglia neurons. J Mol Neurosci. 2010;42:390–6.
Liu F, Cao QH, Lu DJ, Luo B, Lu XF, Luo RC, et al. TMEM16A overexpression contributes to tumor invasion and poor prognosis of human gastric cancer through TGF-β signaling. Oncotarget. 2015;6:11585–99.
Zhou Z, Ji Z, Wang Y, Li J, Cao H, Zhu HH, et al. TRIM59 is upregulated in gastric tumors, promoting ubiquitination and degradation of p53. Gastroenterology. 2014;147:1043–54.
Acknowledgements
This work was supported by the National Key Research and Development Program of China (No. 2016YFC1302200 to HD), National Natural Science Foundation of China (No. 81602577 to BT), and Basic Science and Frontier Technology Research Project of Chongqing (No. cstc2017jcyjAX0149 to BT).
Author contributions
HD and SY conceived of the study; BT and MXZ designed the experiments; BT, JW, XS, JL, RX, TD, and YX performed the experiments; BT and JW performed analysis and interpretation of data; HD and BT wrote the manuscript; MXZ, JMC, and SY critically reviewed the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Tang, B., Wu, J., Zhu, M.X. et al. VPAC1 couples with TRPV4 channel to promote calcium-dependent gastric cancer progression via a novel autocrine mechanism. Oncogene 38, 3946–3961 (2019). https://doi.org/10.1038/s41388-019-0709-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-019-0709-6
This article is cited by
-
Pathological mechanisms of cold and mechanical stress in modulating cancer progression
Human Cell (2024)
-
Crosstalk Between Peripheral Innervation and Pancreatic Ductal Adenocarcinoma
Neuroscience Bulletin (2023)
-
NCX1 coupled with TRPC1 to promote gastric cancer via Ca2+/AKT/β-catenin pathway
Oncogene (2022)
-
Endothelial TRPV4 channels prevent tumor growth and metastasis via modulation of tumor angiogenesis and vascular integrity
Angiogenesis (2021)
-
The role of TRPV1 ion channels in the suppression of gastric cancer development
Journal of Experimental & Clinical Cancer Research (2020)