Abstract
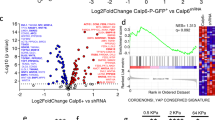
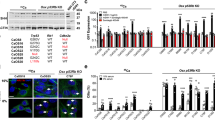
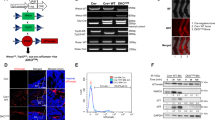
The stem cell transcription factor Sox2 is highly expressed in many cancers where it is thought to mark cancer stem cells (CSCs). In osteosarcomas, the most common bone malignancy, high Sox2 expression marks and maintains a fraction of tumor-initiating cells that show all the properties of CSC. Knockdown of Sox2 expression abolishes tumorigenicity and suppresses the CSC phenotype. Here we show that, in a mouse model of osteosarcoma, osteoblast-specific Sox2 conditional knockout (CKO) causes a drastic reduction in the frequency and onset of tumors. The rare tumors detected in the Sox2 CKO animals were all Sox2 positive, indicating that they arose from cells that had escaped Sox2 deletion. Furthermore, Sox2 inactivation in cultured osteosarcoma cells by CRISPR/CAS technology leads to a loss of viability and proliferation of the entire cell population. Inactivation of the YAP gene, a major Hippo pathway effector which is a direct Sox2 target, causes similar results and YAP overexpression rescues cells from the lethality caused by Sox2 inactivation. These effects were osteosarcoma-specific, suggesting a mechanism of cell “addiction” to Sox2-initiated pathways. The requirement of Sox2 for osteosarcoma formation as well as for the survival of the tumor cells suggests that disruption of Sox2-initiated pathways could be an effective strategy for the treatment of osteosarcoma.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Arnold K, Sarkar A, Yram MA, Polo JM, Bronson R, Senqupta S, et al. Sox2(+) adult stem and progenitor cells are important for tissue regeneration and survival of mice. Cell Stem Cell. 2011;9:317–29.
Avilion AA, Nicolis SK, Pevny LH, Perez L, Vivian N, Lovell-Badge R. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev. 2003;17:126–40.
Basu-Roy U, Basilico C, Mansukhani A. Perspectives on cancer stem cells in osteosarcoma. Cancer Lett. 2013;338:158–67.
Driessens G, Blanpain C. Long live sox2: sox2 lasts a lifetime. Cell Stem Cell. 2011;9:283–4.
Niwa H. How is pluripotency determined and maintained? Development. 2007;134:635–46.
Basu-Roy U, Seo E, Ramanathapuram L, Rapp TB, Perry JA, Orkin SH, et al. Sox2 maintains self renewal of tumor-initiating cells in osteosarcomas. Oncogene. 2012;31:2270–82.
Rainusso N, Wang LL, Yustein JT. The adolescent and young adult with cancer: state of the art -- bone tumors. Curr Oncol Rep. 2013;15:296–307.
Basu-Roy U, Bayin NS, Rattanakorn K, Han E, Placantonakis DG, Mansukhani A, et al. Sox2 antagonizes the Hippo pathway to maintain stemness in cancer cells. Nat Commun. 2015;6:6411.
Yu FX, Guan KL. The Hippo pathway: regulators and regulations. Genes Dev. 2013;27:355–71.
Meng Z, Moroishi T, Guan KL. Mechanisms of Hippo pathway regulation. Genes Dev. 2016;30:1–17.
Moroishi T, Hansen CG, Guan KL. The emerging roles of YAP1 and TAZ in cancer. Nat Rev Cancer. 2015;15:73–9.
Seo E, Basu-Roy U, Gunaratne PH, Coarfa C, Lim DS, Basilico C, et al. SOX2 regulates YAP1 to maintain stemness and determine cell fate in the osteo-adipo lineage. Cell Rep. 2013;3:2075–87.
Berman S, Calo E, Landman AS, Danelian PS, Miller ES, West JC, et al. Metastatic osteosarcoma induced by inactivation of Rb and p53 in the osteoblast lineage. Proc Natl Acad Sci USA. 2008;105:11851–6.
Walkley CR, Qudsi R, Sankaran VG, Perry JA, Gostissa M, Roth SI, et al. Conditional mouse osteosarcoma, dependent on p53 loss and potentiated by loss of Rb, mimics the human disease. Genes Dev. 2008;22:1662–76.
Basu-Roy U, Ambrosetti D, Favaro R, Nicolis SK, Mansukhani A, Basilico C. The transcription factor Sox2 is required for osteoblast self-renewal. Cell Death Differ. 2010;17:1345–53.
Guernet A, Grumolato L. CRISPR/Cas9 editing of the genome for cancer modeling. Methods. 2017;121-122:130–7.
Basilico C, Matsuya Y, Green H. Origin of the thymidine kinase induced by polyoma virus in productively infected cells. J Virol. 1969;3:140–5.
Liao S, Tammaro M, Yan H. Enriching CRISPR-Cas9 targeted cells by co-targeting the HPRT gene. Nucleic Acids Res. 2015;43:e134.
Lian I, Kim J, Okazawa H, Zhao B, Yu J, Chinnayan A, et al. The role of YAP1 transcription coactivator in regulating stem cell self-renewal and differentiation. Genes Dev. 2010;24:1106–18.
Tafani M, Perrone GA, Pucci B, Russo A, Bizzarri M, Mechanick JI, et al. Reprogramming cancer cells in endocrine-related tumors: open issues. Curr Med Chem. 2014;21:1146–51.
Palumbo P, Miconi G, Cinque B, Lombardi F, La Torre C, Dehcordi SR, et al. NOS2 expression in glioma cell lines and glioma primary cell cultures: correlation with neurosphere generation and SOX-2 expression. Oncotarget. 2017;8:25582–98.
Song WS, Yang YP, Huang CS, Lu KH, Liu WH, et al. Sox2, a stemness gene, regulates tumor-initiating and drug-resistant properties in CD133-positive glioblastoma stem cells. J Chin Med Assoc. 2016;79:538–45.
Mansouri S, Nejad R, Karabork M, Ekinci C, Solaroglu I, Aldape KD, et al. Sox2: regulation of expression and contribution to brain tumors. CNS Oncol. 2016;5:159–73.
Santini R, Pietrobono S, Pandolfi S, Montagnani V, D’Amico M, Penachioni JY, et al. SOX2 regulates self-renewal and tumorigenicity of human melanoma-initiating cells. Oncogene. 2014;33:4697–708.
Tan Y, Tajik A, Chen J, Jia Q, Chowdhury F, Wang L, Chen J, et al. Matrix softness regulates plasticity of tumour-repopulating cells via H3K9 demethylation and Sox2 expression. Nat Commun. 2014;5:4619.
Weina K, Wu H, Knappe N, Orouji E, Novak D, Bernhardt M, et al. TGF-β induces SOX2 expression in a time-dependent manner in human melanoma cells. Pigment Cell Melanoma Res. 2016;29:453–8.
Ferone G, Song JY, Sutherland KD, Bhaskaran R, Monkhorst K, Lambooij JP, et al. SOX2 is the determining oncogenic switch in promoting lung squamous cell carcinoma from different cells of origin. Cancer Cell. 2016;30:519–32.
Siegle JM, Basin A, Sastre-Perona A, Yonekubo Y, Brown J, Sennett R, et al. SOX2 is a cancer-specific regulator of tumour initiating potential in cutaneous squamous cell carcinoma. Nat Commun. 2014;5:4511.
Boumahdi S, Driessens G, Lapouge G, Rorive S, Nassar D, Lemercier M, et al. Sox2 controls tumor initiation and cancer stem-cell functions in squamous cell carcinoma. Nature. 2014;511:246–50.
Shahriyari L, Komarova NL. Symmetric vs. asymmetric stem cell divisions: an adaptation against cancer? PLoS ONE. 2013;8:e76195.
Brinkman EK, Chen T, Amendola M, van Steensel B. Easy quantitative assessment of genome editing by sequence trace decomposition. Nucleic Acids Res. 2014;42:e168.
Acknowledgements
This investigation was supported by NYSTEM contract CO29560 and NIH/ NCI-R21CA186031. We thank Dr. Stuart Orkin for providing us with the Tp53, Rb mutant mice; Matthew Murtha for his contribution to some of the initial experiments; and Upal BasuRoy for helpful discussions. We also wish to acknowledge the Histopathology core services at NYU Langone Medical Center.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Maurizi, G., Verma, N., Gadi, A. et al. Sox2 is required for tumor development and cancer cell proliferation in osteosarcoma. Oncogene 37, 4626–4632 (2018). https://doi.org/10.1038/s41388-018-0292-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-018-0292-2
This article is cited by
-
LncRNA WAC-AS1 promotes osteosarcoma Metastasis and stemness by sponging miR-5047 to upregulate SOX2
Biology Direct (2023)
-
A novel molecular classification method for osteosarcoma based on tumor cell differentiation trajectories
Bone Research (2023)
-
SOX2 suppresses osteoblast differentiation of MC3T3-E1 cells through activating the transcription of LGR4
In Vitro Cellular & Developmental Biology - Animal (2023)
-
The role of SPI1-TYROBP-FCER1G network in oncogenesis and prognosis of osteosarcoma, and its association with immune infiltration
BMC Cancer (2022)
-
Deletion of TRIB3 disrupts the tumor progression induced by integrin αvβ3 in lung cancer
BMC Cancer (2022)