Abstract
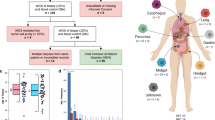
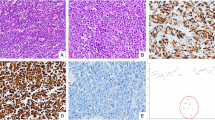
Neuroendocrine bladder cancer is a relatively rare but often lethal malignancy, with cell of origin, oncogenomic architecture and standard treatment poorly defined. Here we performed comprehensive whole-genome and transcriptome sequencing on a unique cohort of genitourinary neuroendocrine neoplasms, mainly small cell carcinomas of the urinary bladder. The mutational landscape and signatures of neuroendocrine bladder cancer strikingly resembled those in conventional urothelial carcinoma, along with typically mixed histologies, supporting a common cellular origin. We identified pervasive age-related and APOBEC-mediated mutagenesis patterns, and one patient displayed a somatic fingerprint attributable to aristolochic acid exposure, an established etiology of urothelial cell carcinoma. Deep RNA sequencing revealed dysregulated tumorigenic pathways and novel fusion transcripts, including a targetable in-frame PVT1-ERBB2 variant associated with aberrant expression of ERBB2 gene (encoding HER2 receptor). Furthermore, we provided preliminary evidence that combined TP53 and RB1 depletion favored lineage switching from oncogene-addicted urothelial cancer cells to neuroendocrine-like tumor cells, and resulted in decreased response to targeted agents. Together, these data present the first high-resolution genomic portrait of neuroendocrine bladder cancer, which holds important implications for the biological understanding and rational treatment of this deadly disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Kouba E, Cheng L. Neuroendocrine tumors of the urinary bladder according to the 2016 World Health Organization classification: molecular and clinical characteristics. Endocr Pathol. 2016;27:188–99.
Kouba EJ, Cheng L. Understanding the genetic landscape of small cell carcinoma of the urinary bladder and implications for diagnosis, prognosis, and treatment: a review. JAMA Oncol. 2017;3:1570–8.
Rickman DS, Beltran H, Demichelis F, Rubin MA. Biology and evolution of poorly differentiated neuroendocrine tumors. Nat Med. 2017;23:1–10.
Cheng L, Jones TD, McCarthy RP, Eble JN, Wang M, MacLennan GT, et al. Molecular genetic evidence for a common clonal origin of urinary bladder small cell carcinoma and coexisting urothelial carcinoma. Am J Pathol. 2005;166:1533–9.
Priemer DS, Wang M, Zhang S, Lopez-Beltran A, Kouba E, Montironi R, et al. Small-cell carcinomas of the urinary bladder and prostate: TERT promoter mutation status differentiates sites of malignancy and provides evidence of common clonality between small-cell carcinoma of the urinary bladder and urothelial carcinoma. Eur Urol Focus 2017.
Gui Y, Guo G, Huang Y, Hu X, Tang A, Gao S, et al. Frequent mutations of chromatin remodeling genes in transitional cell carcinoma of the bladder. Nat Genet. 2011;43:875–8.
Cancer Genome Atlas Research N. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014;507:315–22.
Poon SL, Pang ST, McPherson JR, Yu W, Huang KK, Guan P, et al. Genome-wide mutational signatures of aristolochic acid and its application as a screening tool. Sci Transl Med. 2013;5:197ra101.
Hoang ML, Chen CH, Sidorenko VS, He J, Dickman KG, Yun BH, et al. Mutational signature of aristolochic acid exposure as revealed by whole-exome sequencing. Sci Transl Med. 2013;5:197ra102.
Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541:321–30.
Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;3:75ra26.
Niederst MJ, Sequist LV, Poirier JT, Mermel CH, Lockerman EL, Garcia AR, et al. RB loss in resistant EGFR mutant lung adenocarcinomas that transform to small-cell lung cancer. Nat Commun. 2015;6:6377.
Mu P, Zhang Z, Benelli M, Karthaus WR, Hoover E, Chen CC, et al. SOX2 promotes lineage plasticity and antiandrogen resistance in TP53- and RB1-deficient prostate cancer. Science. 2017;355:84–88.
Ku SY, Rosario S, Wang Y, Mu P, Seshadri M, Goodrich ZW, et al. Rb1 and Trp53 cooperate to suppress prostate cancer lineage plasticity, metastasis, and antiandrogen resistance. Science. 2017;355:78–83.
Chang MT, Penson AV, Desai NB, Socci ND, Shen R, Seshan V, et al. Small cell carcinomas of the bladder and lung are characterized by a convergent but distinct pathogenesis. Clin Cancer Res. 2017.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81672514 to HC; 81472537 and 81672714 to GZ; 81502597 to YJ), Shanghai Natural Science Foundation (16ZR1420300 to HC), Foundation of Shanghai Hospital Development Center (SHDC12015125 to HC), the Grants from the State Key Laboratory of Oncogenes and Related Genes (no. 91-15-12 to GZ; SB17-06 to M-CC), Shanghai Municipal Education Commission-Gaofeng Clinical Medicine Grant Support (20161313 to GZ), the Shanghai Institutions of Higher Learning (Eastern Scholar to GZ), Shanghai Rising-Star Program (16QA1403600 to GZ), Shanghai Municipal Commission of Health and Family Planning (20174Y0189 to YJ and 20174Y0043 to M-CC).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
These authors contributed equally: Peiye Shen, Ying Jing, Ruiyun Zhang.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Shen, P., Jing, Y., Zhang, R. et al. Comprehensive genomic profiling of neuroendocrine bladder cancer pinpoints molecular origin and potential therapeutics. Oncogene 37, 3039–3044 (2018). https://doi.org/10.1038/s41388-018-0192-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-018-0192-5
This article is cited by
-
Genetic and immunohistochemical profiling of small cell and large cell neuroendocrine carcinomas of the breast
Modern Pathology (2022)
-
Application of the CRISPR/Cas9-based gene editing technique in basic research, diagnosis, and therapy of cancer
Molecular Cancer (2021)
-
Small cell carcinoma of the bladder: the characteristics of molecular alterations, treatment, and follow-up
Medical Oncology (2019)
-
Long non-coding RNA PVT1 interacts with MYC and its downstream molecules to synergistically promote tumorigenesis
Cellular and Molecular Life Sciences (2019)
-
Neuroendocrine disease genomics
Nature Reviews Urology (2018)