Abstract
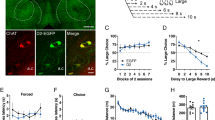
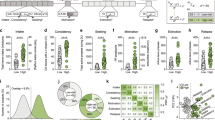
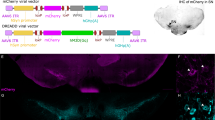
Impulsive choice has enduring trait-like characteristics and is defined by preference for small immediate rewards over larger delayed ones. Importantly, it is a determining factor in the development and persistence of substance use disorder (SUD). Emerging evidence from human and animal studies suggests frontal cortical regions exert influence over striatal reward processing areas during decision-making in impulsive choice or delay discounting (DD) tasks. The goal of this study was to examine how these circuits are involved in decision-making in animals with defined trait impulsivity. To this end, we trained adolescent male rats to stable behavior on a DD procedure and then re-trained them in adulthood to assess trait-like, conserved impulsive choice across development. We then used chemogenetic tools to selectively and reversibly target corticostriatal projections during performance of the DD task. The prelimbic region of the medial prefrontal cortex (mPFC) was injected with a viral vector expressing inhibitory designer receptors exclusively activated by designer drugs (Gi-DREADD), and then mPFC projections to the nucleus accumbens core (NAc) were selectively suppressed by intra-NAc administration of the Gi-DREADD actuator clozapine-n-oxide (CNO). Inactivation of the mPFC-NAc projection elicited a robust increase in impulsive choice in rats with lower vs. higher baseline impulsivity. This demonstrates a fundamental role for mPFC afferents to the NAc during choice impulsivity and suggests that maladaptive hypofrontality may underlie decreased executive control in animals with higher levels of choice impulsivity. Results such as these may have important implications for the pathophysiology and treatment of impulse control, SUDs, and related psychiatric disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 13 print issues and online access
$259.00 per year
only $19.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Chung SH, Herrnstein RJ. Choice and delay of reinforcement. J Exp Anal Behav. 1967;10:67–74.
Evenden JL, Ryan CN. The pharmacology of impulsive behaviour in rats VI: the effects of ethanol and selective serotonergic drugs on response choice with varying delays of reinforcement. Psychopharmacol (Berl). 1999;146:413–21.
Lempert KM, Steinglass JE, Pinto A, Kable JW, Simpson HB. Can delay discounting deliver on the promise of RDoC? Psychol Med. 2019;49:190–99.
Vanderveldt A, Oliveira L, Green L. Delay discounting: pigeon, rat, human–does it matter? J Exp Psychol Anim Learn Cogn. 2016;42:141–62.
Mitchell SH. Linking delay discounting and substance use disorders: genotypes and phenotypes. Perspect Behav Sci. 2019;42:419–32.
Amlung M, Marsden E, Holshausen K, Morris V, Patel H, Vedelago L, et al. Delay discounting as a transdiagnostic process in psychiatric disorders: a meta-analysis. JAMA Psychiatry. 2019;76:1176–86.
Kirby KN, Petry NM, Bickel WK. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J Exp Psychol Gen. 1999;128:78–87.
Mies GW, de Water E, Wiersema JR, Scheres A. Delay discounting of monetary gains and losses in adolescents with ADHD: contribution of delay aversion to choice. Child Neuropsychol. 2019;25:528–47.
Petry NM, Casarella T. Excessive discounting of delayed rewards in substance abusers with gambling problems. Drug Alcohol Depend. 1999;56:25–32.
Anker JJ, Perry JL, Gliddon LA, Carroll ME. Impulsivity predicts the escalation of cocaine self-administration in rats. Pharm Biochem Behav. 2009;93:343–8.
Athamneh LN, Freitas Lemos R, Basso JC, Tomlinson DC, Craft WH, Stein MD, et al. The phenotype of recovery II: The association between delay discounting, self-reported quality of life, and remission status among individuals in recovery from substance use disorders. Exp Clin Psychopharmacol. 2022;30:59–72.
Kraplin A, Hofler M, Pooseh S, Wolff M, Kronke KM, Goschke T, et al. Impulsive decision-making predicts the course of substance-related and addictive disorders. Psychopharmacol (Berl). 2020;237:2709–24.
Perry JL, Larson EB, German JP, Madden GJ, Carroll ME. Impulsivity (delay discounting) as a predictor of acquisition of IV cocaine self-administration in female rats. Psychopharmacol (Berl). 2005;178:193–201.
Haynes JM, Galizio A, Frye CCJ, Towse CC, Morrissey KN, Serang S, et al. Discounting of food and water in rats shows trait- and state-like characteristics. J Exp Anal Behav. 2021;115:495–509.
Odum AL, Baumann AAL. Delay discounting: state and trait variable. In: Madden G, Bickel W, editors. The behavioral and neurological science of discounting. Washington, DC: APA Books; 2010. p. 39–65.
Anokhin AP, Golosheykin S, Grant JD, Heath AC. Heritability of delay discounting in adolescence: a longitudinal twin study. Behav Genet. 2011;41:175–83.
Anokhin AP, Grant JD, Mulligan RC, Heath AC. The genetics of impulsivity: evidence for the heritability of delay discounting. Biol Psychiatry. 2015;77:887–94.
Isen JD, Sparks JC, Iacono WG. Predictive validity of delay discounting behavior in adolescence: a longitudinal twin study. Exp Clin Psychopharmacol. 2014;22:434–43.
Odum AL. Delay discounting: trait variable? Behav Process. 2011;87:1–9.
Odum AL, Becker RJ, Haynes JM, Galizio A, Frye CCJ, Downey H, et al. Delay discounting of different outcomes: review and theory. J Exp Anal Behav. 2020;113:657–79.
Peterson JR, Hill CC, Kirkpatrick K. Measurement of impulsive choice in rats: same- and alternate-form test-retest reliability and temporal tracking. J Exp Anal Behav. 2015;103:166–79.
Weafer J, Baggott MJ, de Wit H. Test-retest reliability of behavioral measures of impulsive choice, impulsive action, and inattention. Exp Clin Psychopharmacol. 2013;21:475–81.
Craig AR, Maxfield AD, Stein JS, Renda CR, Madden GJ. Do the adjusting-delay and increasing-delay tasks measure the same construct: delay discounting? Behav Pharm. 2014;25:306–15.
Epstein LH, Richards JB, Saad FG, Paluch RA, Roemmich JN, Lerman C. Comparison between two measures of delay discounting in smokers. Exp Clin Psychopharmacol. 2003;11:131–8.
Madden GJ, Petry NM, Badger GJ, Bickel WK. Impulsive and self-control choices in opioid-dependent patients and non-drug-using control participants: drug and monetary rewards. Exp Clin Psychopharmacol. 1997;5:256–62.
de Wit H. Acute administration of d-amphetamine decreases impulsivity in healthy volunteers. Neuropsychopharmacology. 2002;27:813–25.
Diller JW, Saunders BT, Anderson KG. Effects of acute and repeated administration of caffeine on temporal discounting in rats. Pharm Biochem Behav. 2008;89:546–55.
Smethells JR, Carroll ME. Discrepant effects of acute cocaine on impulsive choice (delay discounting) in female rats during an increasing- and adjusting-delay procedure. Psychopharmacol (Berl). 2015;232:2455–62.
Doremus-Fitzwater TL, Barreto M, Spear LP. Age-related differences in impulsivity among adolescent and adult Sprague-Dawley rats. Behav Neurosci. 2012;126:735–41.
Green L, Myerson J, Ostaszewski P. Discounting of delayed rewards across the life span: age differences in individual discounting functions. Behav Process. 1999;46:89–96.
Leverett S, Garza C, Seaman K. The effect of delay duration on delay discounting across adulthood. J Gerontol B Psychol Sci Soc Sci. 2022;77:467–71.
Lukkes JL, Thompson BS, Freund N, Andersen SL. The developmental inter-relationships between activity, novelty preferences, and delay discounting in male and female rats. Dev Psychobiol. 2016;58:231–42.
Olson EA, Hooper CJ, Collins P, Luciana M. Adolescents’ performance on delay and probability discounting tasks: contributions of age, intelligence, executive functioning, and self-reported externalizing behavior. Pers Individ Dif. 2007;43:1886–97.
Pinkston JW, Lamb RJ. Delay discounting in C57BL/6J and DBA/2J mice: adolescent-limited and life-persistent patterns of impulsivity. Behav Neurosci. 2011;125:194–201.
Roesch MR, Bryden DW, Cerri DH, Haney ZR, Schoenbaum G. Willingness to wait and altered encoding of time-discounted reward in the orbitofrontal cortex with normal aging. J Neurosci. 2012;32:5525–33.
Simon NW, LaSarge CL, Montgomery KS, Williams MT, Mendez IA, Setlow B, et al. Good things come to those who wait: attenuated discounting of delayed rewards in aged Fischer 344 rats. Neurobiol Aging. 2010;31:853–62.
Hammerslag LR, Belagodu AP, Aladesuyi Arogundade OA, Karountzos AG, Guo Q, Galvez R, et al. Adolescent impulsivity as a sex-dependent and subtype-dependent predictor of impulsivity, alcohol drinking and dopamine D(2) receptor expression in adult rats. Addict Biol. 2019;24:193–205.
Macedo I, Fernandes C, Barbosa F, Marques-Teixeira J. Delay discounting in aging: the influence of cognitive and psychological variables. Behav Neurosci. 2022;136:392–403.
Robertson SH, Rasmussen EB. Effects of a cafeteria diet on delay discounting in adolescent and adult rats: alterations on dopaminergic sensitivity. J Psychopharmacol. 2017;31:1419–29.
Steward T, Mestre-Bach G, Fernandez-Aranda F, Granero R, Perales JC, Navas JF, et al. Delay discounting and impulsivity traits in young and older gambling disorder patients. Addict Behav. 2017;71:96–103.
McClure J, Podos J, Richardson HN. Isolating the delay component of impulsive choice in adolescent rats. Front Integr Neurosci. 2014;8:3.
Chen Z, Becker B, Qin P, Lei W, Chen J, Liu P, et al. Neural networks during delay discounting as trans-disease marker: a meta-analytical review. J Psychiatr Res. 2021;139:62–70.
McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503–7.
Peters J, Buchel C. Episodic future thinking reduces reward delay discounting through an enhancement of prefrontal-mediotemporal interactions. Neuron. 2010;66:138–48.
Schmaal L, Goudriaan AE, van der Meer J, van den Brink W, Veltman DJ. The association between cingulate cortex glutamate concentration and delay discounting is mediated by resting state functional connectivity. Brain Behav. 2012;2:553–62.
Hampton WH, Alm KH, Venkatraman V, Nugiel T, Olson IR. Dissociable frontostriatal white matter connectivity underlies reward and motor impulsivity. NeuroImage. 2017;150:336–43.
Peper JS, Mandl RC, Braams BR, de Water E, Heijboer AC, Koolschijn PC, et al. Delay discounting and frontostriatal fiber tracts: a combined DTI and MTR study on impulsive choices in healthy young adults. Cereb Cortex. 2013;23:1695–702.
Wang Q, Lv C, He Q, Xue G. Dissociable fronto-striatal functional networks predict choice impulsivity. Brain Struct Funct. 2020;225:2377–86.
Bezzina G, Cheung TH, Asgari K, Hampson CL, Body S, Bradshaw CM, et al. Effects of quinolinic acid-induced lesions of the nucleus accumbens core on inter-temporal choice: a quantitative analysis. Psychopharmacol (Berl). 2007;195:71–84.
Cardinal RN, Pennicott DR, Sugathapala CL, Robbins TW, Everitt BJ. Impulsive choice induced in rats by lesions of the nucleus accumbens core. Science. 2001;292:2499–501.
da Costa Araujo S, Body S, Hampson CL, Langley RW, Deakin JF, Anderson IM, et al. Effects of lesions of the nucleus accumbens core on inter-temporal choice: further observations with an adjusting-delay procedure. Behav Brain Res. 2009;202:272–7.
Galtress T, Kirkpatrick K. The role of the nucleus accumbens core in impulsive choice, timing, and reward processing. Behav Neurosci. 2010;124:26–43.
Moschak TM, Mitchell SH. Partial inactivation of nucleus accumbens core decreases delay discounting in rats without affecting sensitivity to delay or magnitude. Behav Brain Res. 2014;268:159–68.
Orsini CA, Mitchell MR, Heshmati SC, Shimp KG, Spurrell MS, Bizon JL, et al. Effects of nucleus accumbens amphetamine administration on performance in a delay discounting task. Behav Brain Res. 2017;321:130–36.
Steele CC, Peterson JR, Marshall AT, Stuebing SL, Kirkpatrick K. Nucleus accumbens core lesions induce sub-optimal choice and reduce sensitivity to magnitude and delay in impulsive choice tasks. Behav Brain Res. 2018;339:28–38.
Tedford SE, Persons AL, Napier TC. Dopaminergic lesions of the dorsolateral striatum in rats increase delay discounting in an impulsive choice task. PLoS One. 2015;10:e0122063.
Valencia-Torres L, Olarte-Sanchez CM, da Costa Araujo S, Body S, Bradshaw CM, Szabadi E. Nucleus accumbens and delay discounting in rats: evidence from a new quantitative protocol for analysing inter-temporal choice. Psychopharmacol (Berl). 2012;219:271–83.
Yates JR, Bardo MT. Effects of intra-accumbal administration of dopamine and ionotropic glutamate receptor drugs on delay discounting performance in rats. Behav Neurosci. 2017;131:392–405.
Cardinal RN, Parkinson JA, Marbini HD, Toner AJ, Bussey TJ, Robbins TW, et al. Role of the anterior cingulate cortex in the control over behavior by Pavlovian conditioned stimuli in rats. Behav Neurosci. 2003;117:566–87.
Zhang R, Chen Z, Xu T, Feng T. The neural basis underlying the relation between the action identification level and delay discounting: The medial and orbital frontal cortex functional connectivity with the precuneus. Int J Psychophysiol. 2021;159:74–82.
Sellitto M, Ciaramelli E, di Pellegrino G. Myopic discounting of future rewards after medial orbitofrontal damage in humans. J Neurosci. 2010;30:16429–36.
Seamans JK, Lapish CC, Durstewitz D. Comparing the prefrontal cortex of rats and primates: insights from electrophysiology. Neurotox Res. 2008;14:249–62.
Laubach M, Amarante LM, Swanson K, White SR. What, if anything, is rodent prefrontal cortex? eNeuro. 2018;5:ENEURO.0315-18.2018.
Heilbronner SR, Rodriguez-Romaguera J, Quirk GJ, Groenewegen HJ, Haber SN. Circuit-based corticostriatal homologies between rat and primate. Biol Psychiatry. 2016;80:509–21.
Zeeb FD, Floresco SB, Winstanley CA. Contributions of the orbitofrontal cortex to impulsive choice: interactions with basal levels of impulsivity, dopamine signalling, and reward-related cues. Psychopharmacol (Berl). 2010;211:87–98.
Winstanley CA, Theobald DE, Cardinal RN, Robbins TW. Contrasting roles of basolateral amygdala and orbitofrontal cortex in impulsive choice. J Neurosci. 2004;24:4718–22.
Stopper CM, Green EB, Floresco SB. Selective involvement by the medial orbitofrontal cortex in biasing risky, but not impulsive, choice. Cereb Cortex. 2014;24:154–62.
Mobini S, Body S, Ho MY, Bradshaw CM, Szabadi E, Deakin JF, et al. Effects of lesions of the orbitofrontal cortex on sensitivity to delayed and probabilistic reinforcement. Psychopharmacol (Berl). 2002;160:290–8.
Mar AC, Walker AL, Theobald DE, Eagle DM, Robbins TW. Dissociable effects of lesions to orbitofrontal cortex subregions on impulsive choice in the rat. J Neurosci. 2011;31:6398–404.
Kheramin S, Body S, Ho MY, Velázquez-Martinez DN, Bradshaw CM, Szabadi E, et al. Role of the orbital prefrontal cortex in choice between delayed and uncertain reinforcers: a quantitative analysis. Behav Process. 2003;64:239–50.
Jo S, Kim KU, Lee D, Jung MW. Effect of orbitofrontal cortex lesions on temporal discounting in rats. Behav Brain Res. 2013;245:22–8.
Freund N, MacGillivilray HT, Thompson BS, Lukkes JL, Stanis JJ, Brenhouse HC, et al. Sex-dependent changes in ADHD-like behaviors in juvenile rats following cortical dopamine depletion. Behav Brain Res. 2014;270:357–63.
Yates JR, Perry JL, Meyer AC, Gipson CD, Charnigo R, Bardo MT. Role of medial prefrontal and orbitofrontal monoamine transporters and receptors in performance in an adjusting delay discounting procedure. Brain Res. 2014;1574:26–36.
Sonntag KC, Brenhouse HC, Freund N, Thompson BS, Puhl M, Andersen SL. Viral over-expression of D1 dopamine receptors in the prefrontal cortex increase high-risk behaviors in adults: comparison with adolescents. Psychopharmacol (Berl). 2014;231:1615–26.
Feja M, Koch M. Ventral medial prefrontal cortex inactivation impairs impulse control but does not affect delay-discounting in rats. Behav Brain Res. 2014;264:230–9.
Deziel RA, Tasker RA. Effects of endothelin-induced prefrontal cortical lesions on delay discounting in the rat. Behav Neurosci. 2017;131:11–19.
Churchwell JC, Morris AM, Heurtelou NM, Kesner RP. Interactions between the prefrontal cortex and amygdala during delay discounting and reversal. Behav Neurosci. 2009;123:1185–96.
Sackett DA, Moschak TM, Carelli RM. Prelimbic cortical neurons track preferred reward value and reflect impulsive choice during delay discounting behavior. J Neurosci. 2019;39:3108–18.
Doidge JL, Flora DB, Toplak ME. A meta-analytic review of sex differences on delay of gratification and temporal discounting tasks in ADHD and typically developing samples. J Atten Disord. 2021;25:540–61.
Panfil K, Bailey C, Davis I, Mains A, Kirkpatrick K. A time-based intervention to treat impulsivity in male and female rats. Behav Brain Res. 2020;379:112316.
Myerson J, Green L, Warusawitharana M. Area under the curve as a measure of discounting. J Exp Anal Behav. 2001;76:235–43.
Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci USA. 2007;104:5163–8.
Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 7th ed. London: Academic Press. 2013.
Voorn P, Vanderschuren LJ, Groenewegen HJ, Robbins TW, Pennartz CM. Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci. 2004;27:468–74.
Gorelova N, Yang CR. The course of neural projection from the prefrontal cortex to the nucleus accumbens in the rat. Neuroscience. 1997;76:689–706.
Montaron MF, Deniau JM, Menetrey A, Glowinski J, Thierry AM. Prefrontal cortex inputs of the nucleus accumbens-nigro-thalamic circuit. Neuroscience. 1996;71:371–82.
Roth BL. DREADDs for neuroscientists. Neuron. 2016;89:683–94.
Gomez JL, Bonaventura J, Lesniak W, Mathews WB, Sysa-Shah P, Rodriguez LA, et al. Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science. 2017;357:503–07.
Tian L, Liu X, Mei X, Cui R, Li X. The role of dopamine D1- and D2-like receptors related to muscarinic M1 receptors in impulsive choice in high-impulsive and low-impulsive rats. Pharm Biochem Behav. 2019;176:43–52.
Moreno M, Azocar V, Verges A, Fuentealba JA. High impulsive choice is accompanied by an increase in dopamine release in rat dorsolateral striatum. Behav Brain Res. 2021;405:113199.
Marusich JA, Bardo MT. Differences in impulsivity on a delay-discounting task predict self-administration of a low unit dose of methylphenidate in rats. Behav Pharm. 2009;20:447–54.
Anker JJ, Zlebnik NE, Gliddon LA, Carroll ME. Performance under a Go/No-go task in rats selected for high and low impulsivity with a delay-discounting procedure. Behav Pharm. 2009;20:406–14.
Stein JS, Johnson PS, Renda CR, Smits RR, Liston KJ, Shahan TA, et al. Early and prolonged exposure to reward delay: effects on impulsive choice and alcohol self-administration in male rats. Exp Clin Psychopharmacol. 2013;21:172–80.
Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–63.
Sengupta P. The laboratory rat: relating its age with human’s. Int J Prev Med. 2013;4:624–30.
McCutcheon JE, Marinelli M. Age matters. Eur J Neurosci. 2009;29:997–1014.
Keidel K, Rramani Q, Weber B, Murawski C, Ettinger U. Individual differences in intertemporal choice. Front Psychol. 2021;12:643670.
Giertler C, Bohn I, Hauber W. The rat nucleus accumbens is involved in guiding of instrumental responses by stimuli predicting reward magnitude. Eur J Neurosci. 2003;18:1993–6.
Bezzina G, Body S, Cheung TH, Hampson CL, Bradshaw CM, Szabadi E, et al. Effect of disconnecting the orbital prefrontal cortex from the nucleus accumbens core on inter-temporal choice behaviour: a quantitative analysis. Behav Brain Res. 2008;191:272–9.
Li B, Nguyen TP, Ma C, Dan Y. Inhibition of impulsive action by projection-defined prefrontal pyramidal neurons. Proc Natl Acad Sci USA. 2020;117:17278–87.
Cardinal RN, Cheung TH. Nucleus accumbens core lesions retard instrumental learning and performance with delayed reinforcement in the rat. BMC Neurosci. 2005;6:9.
Cardinal RN, Parkinson JA, Lachenal G, Halkerston KM, Rudarakanchana N, Hall J, et al. Effects of selective excitotoxic lesions of the nucleus accumbens core, anterior cingulate cortex, and central nucleus of the amygdala on autoshaping performance in rats. Behav Neurosci. 2002;116:553–67.
Basar K, Sesia T, Groenewegen H, Steinbusch HW, Visser-Vandewalle V, Temel Y. Nucleus accumbens and impulsivity. Prog Neurobiol. 2010;92:533–57.
Pothuizen HH, Jongen-Relo AL, Feldon J, Yee BK. Double dissociation of the effects of selective nucleus accumbens core and shell lesions on impulsive-choice behaviour and salience learning in rats. Eur J Neurosci. 2005;22:2605–16.
Feja M, Hayn L, Koch M. Nucleus accumbens core and shell inactivation differentially affects impulsive behaviours in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2014;54:31–42.
Peters J, Buchel C. The neural mechanisms of inter-temporal decision-making: understanding variability. Trends Cogn Sci. 2011;15:227–39.
Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol Behav. 2005;86:773–95.
Wittmann M, Paulus MP. Decision making, impulsivity and time perception. Trends Cogn Sci. 2008;12:7–12.
Baumann AA, Odum AL. Impulsivity, risk taking, and timing. Behav Process. 2012;90:408–14.
Marshall AT, Smith AP, Kirkpatrick K. Mechanisms of impulsive choice: I. Individual differences in interval timing and reward processing. J Exp Anal Behav. 2014;102:86–101.
van den Broek MD, Bradshaw CM, Szabadi E. Performance of normal adults on the matching familiar figures test. Br J Clin Psychol. 1987;26:71–2.
Buhusi CV, Reyes MB, Gathers CA, Oprisan SA, Buhusi M. Inactivation of the medial-prefrontal cortex impairs interval timing precision, but not timing accuracy or scalar timing in a peak-interval procedure in rats. Front Integr Neurosci. 2018;12:20.
Narayanan NS, Horst NK, Laubach M. Reversible inactivations of rat medial prefrontal cortex impair the ability to wait for a stimulus. Neuroscience 2006;139:865–76.
Kim S, Hwang J, Lee D. Prefrontal coding of temporally discounted values during intertemporal choice. Neuron 2008;59:161–72.
Kim S, Hwang J, Seo H, Lee D. Valuation of uncertain and delayed rewards in primate prefrontal cortex. Neural Netw. 2009;22:294–304.
Moschak TM, Carelli RM. Impulsive rats exhibit blunted dopamine release dynamics during a delay discounting task independent of cocaine history. eNeuro. 2017;4:ENEURO.0119-17.2017.
Roesch MR, Singh T, Brown PL, Mullins SE, Schoenbaum G. Ventral striatal neurons encode the value of the chosen action in rats deciding between differently delayed or sized rewards. J Neurosci. 2009;29:13365–76.
Saddoris MP, Sugam JA, Stuber GD, Witten IB, Deisseroth K, Carelli RM. Mesolimbic dopamine dynamically tracks, and is causally linked to, discrete aspects of value-based decision making. Biol Psychiatry. 2015;77:903–11.
Galtress T, Garcia A, Kirkpatrick K. Individual differences in impulsive choice and timing in rats. J Exp Anal Behav. 2012;98:65–87.
Barlow RL, Gorges M, Wearn A, Niessen HG, Kassubek J, Dalley JW, et al. Ventral striatal D2/3 receptor availability is associated with impulsive choice behavior as well as limbic corticostriatal connectivity. Int J Neuropsychopharmacol. 2018;21:705–15.
Laguna A, Lajud N, Juarez J, Sanz-Martin A. Chronic early-life stress increases cognitive impulsivity and D2 immunoreactivity in the nucleus accumbens of adult rats. Dev Psychobiol. 2022;64:e22259.
Simon NW, Beas BS, Montgomery KS, Haberman RP, Bizon JL, Setlow B. Prefrontal cortical-striatal dopamine receptor mRNA expression predicts distinct forms of impulsivity. Eur J Neurosci. 2013;37:1779–88.
Volkow ND, Wang GJ, Fowler JS, Telang F. Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Philos Trans R Soc Lond B Biol Sci. 2008;363:3191–200.
Wang GJ, Volkow ND, Thanos PK, Fowler JS. Similarity between obesity and drug addiction as assessed by neurofunctional imaging: a concept review. J Addict Dis. 2004;23:39–53.
Mazur JE. Choice, delay, probability, and conditioned reinforcement. Anim Learn Behav. 1997;25:131–47.
Williams BA, Dunn R. Preference for conditioned reinforcement. J Exp Anal Behav. 1991;55:37–46.
Robles E, Vargas PA, Bejarano R. Within-subject differences in degree of delay discounting as a function of order of presentation of hypothetical cash rewards. Behav Process. 2009;81:260–3.
Robles E, Vargas PA. Parameters of delay discounting assessment: number of trials, effort, and sequential effects. Behav Process. 2008;78:285–90.
Fox AT, Hand DJ, Reilly MP. Impulsive choice in a rodent model of attention-deficit/hyperactivity disorder. Behav Brain Res. 2008;187:146–52.
Sjoberg E, Ottasen HM, Wilner RG, Johansen EB. Previous experience with delays affects delay discounting in animal model of ADHD. Behav Brain Funct. 2023;19:4.
Slezak JM, Anderson KG. Effects of variable training, signaled and unsignaled delays, and d-amphetamine on delay-discounting functions. Behav Pharm. 2009;20:424–36.
Tanno T, Maguire DR, Henson C, France CP. Effects of amphetamine and methylphenidate on delay discounting in rats: interactions with order of delay presentation. Psychopharmacol (Berl). 2014;231:85–95.
Maguire DR, Henson C, France CP. Effects of amphetamine on delay discounting in rats depend upon the manner in which delay is varied. Neuropharmacology. 2014;87:173–9.
Orsini CA, Setlow B. Sex differences in animal models of decision making. J Neurosci Res. 2017;95:260–69.
Logue AW, Anderson YD. Higher-education administrators: when the future does not make a difference. Psychol Sci. 2001;12:276–81.
Reynolds B, Richards JB, de Wit H. Acute-alcohol effects on the Experiential Discounting Task (EDT) and a question-based measure of delay discounting. Pharm Biochem Behav. 2006;83:194–202.
Smith CL, Hantula DA. Methodological considerations in the study of delay discounting in intertemporal choice: a comparison of tasks and modes. Behav Res Methods. 2008;40:940–53.
Beck RC, Triplett MF. Test-retest reliability of a group-administered paper-pencil measure of delay discounting. Exp Clin Psychopharmacol. 2009;17:345–55.
Kirby KN, Marakovic NN. Delay-discounting probabilistic rewards: rates decrease as amounts increase. Psychon Bull Rev. 1996;3:100–4.
Mischel W, Underwood B. Instrumental ideation in delay of gratification. Child Dev. 1974;45:1083–8.
Eubig PA, Noe TE, Floresco SB, Sable JJ, Schantz SL. Sex differences in response to amphetamine in adult Long-Evans rats performing a delay-discounting task. Pharm Biochem Behav. 2014;118:1–9.
Perry JL, Nelson SE, Anderson MM, Morgan AD, Carroll ME. Impulsivity (delay discounting) for food and cocaine in male and female rats selectively bred for high and low saccharin intake. Pharm Biochem Behav. 2007;86:822–37.
Oh H, Gosnell S, Nguyen T, Tran T, Kosten TR, Salas R. Cingulate cortex structural alterations in substance use disorder psychiatric inpatients. Am J Addict. 2021;30:72–79.
Camchong J, Macdonald AW 3rd, Mueller BA, Nelson B, Specker S, Slaymaker V, et al. Changes in resting functional connectivity during abstinence in stimulant use disorder: a preliminary comparison of relapsers and abstainers. Drug Alcohol Depend. 2014;139:145–51.
Kearns AM, Siemsen BM, Hopkins JL, Weber RA, Scofield MD, Peters J, et al. Chemogenetic inhibition of corticostriatal circuits reduces cued reinstatement of methamphetamine seeking. Addict Biol. 2022;27:e13097.
Kerstetter KA, Wunsch AM, Nakata KG, Donckels E, Neumaier JF, Ferguson SM. Corticostriatal afferents modulate responsiveness to psychostimulant drugs and drug-associated stimuli. Neuropsychopharmacology. 2016;41:1128–37.
Stefanik MT, Moussawi K, Kupchik YM, Smith KC, Miller RL, Huff ML, et al. Optogenetic inhibition of cocaine seeking in rats. Addict Biol. 2013;18:50–3.
Acknowledgements
The authors are grateful for the support and technical expertise of Iness Gildish, Autumn Bows, and Brian Donnellan.
Funding
This work supported by National Institute on Drug Abuse grants R01 DA022340 and R01 DA042595 (JFC), F32 DA039690 (JMW), F32 DA043967 and K99 DA047419 (NEZ), and National Institute on Alcohol Abuse and Alcoholism grant F31 AA024683A (MHP).
Author information
Authors and Affiliations
Contributions
JMW, NEZ, and JFC conceived of and designed the study; JMW, NEZ, VMA, HMD, and MHP carried out the experiments; JRS, LYZ, BNM, and JFC provided essential intellectual input on study design, analysis, and data interpretation; JMW, NEZ, and JRS analyzed the data; JMW and NEZ wrote the initial draft of the manuscript; all authors contributed to, edited, and approved of the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wenzel, J.M., Zlebnik, N.E., Patton, M.H. et al. Selective chemogenetic inactivation of corticoaccumbal projections disrupts trait choice impulsivity. Neuropsychopharmacol. 48, 1821–1831 (2023). https://doi.org/10.1038/s41386-023-01604-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-023-01604-5