Abstract
Binge drinking is a significant problem in adolescent populations, and because of the reciprocal interactions between ethanol (EtOH) consumption and the endocannabinoid (eCB) system, we sought to determine if adolescent EtOH intake altered the localization and function of the cannabinoid 1 (CB1) receptors in the adult brain. Adolescent mice were exposed to a 4-day-per week drinking in the dark (DID) procedure for a total of 4 weeks and then tested after a 2-week withdrawal period. Field excitatory postsynaptic potentials (fEPSPs), evoked by medial perforant path (MPP) stimulation in the dentate gyrus molecular layer (DGML), were significantly smaller. Furthermore, unlike control animals, CB1 receptor activation did not depress fEPSPs in the EtOH-exposed animals. We also examined a form of excitatory long-term depression that is dependent on CB1 receptors (eCB-eLTD) and found that it was completely lacking in the animals that consumed EtOH during adolescence. Histological analyses indicated that adolescent EtOH intake significantly reduced the CB1 receptor distribution and proportion of immunopositive excitatory synaptic terminals in the medial DGML. Furthermore, there was decreased binding of [35S]guanosine-5*-O-(3-thiotriphosphate) ([35S] GTPγS) and the guanine nucleotide-binding (G) protein Gαi2 subunit in the EtOH-exposed animals. Associated with this, there was a significant increase in monoacylglycerol lipase (MAGL) mRNA and protein in the hippocampus of EtOH-exposed animals. Conversely, deficits in eCB-eLTD and recognition memory could be rescued by inhibiting MAGL with JZL184. These findings indicate that repeated exposure to EtOH during adolescence leads to long-term deficits in CB1 receptor expression, eCB-eLTD, and reduced recognition memory, but that these functional deficits can be restored by treatments that increase endogenous 2-arachidonoylglycerol.
Similar content being viewed by others
Introduction
Adolescence is a period of significant growth and development for the brain [1], and EtOH intake during this period can have significant long-term consequences [2]. Alcoholism is a significant problem worldwide, and among alcohol users, adolescents are significantly more likely to engage in a pattern of alcohol use known as “binge drinking”. This is a repetitive pattern of drinking that is characterized by heavy alcohol use in a short period of time, followed by a period of abstinence. Blood ethanol concentrations (BEC) greater than 0.08 g/dL can be obtained by binge drinking, and this has been calculated to correspond to an average adolescent male consuming more than five drinks over a period of 2 h [3].
The adolescent brain is also going through a significant period of maturation that requires changes in neurotransmission, synaptic plasticity, and structural changes in brain regions related to learning, memory, and executive function. Previous studies have demonstrated that binge drinking can adversely affect synaptic transmission and neuroplasticity, and it is believed that these deficits also play a role in the cognitive and emotional issues associated with binge drinking [2, 4,5,6,7,8,9,10]. In the past decade, a developmental role for endocannabinoid receptors has emerged in the adolescent brain [11]. The eCB system is composed of G-protein-coupled cannabinoid receptors (CB1, CB2, and others), endogenous cannabinoids (mainly 2-arachidonoylglycerol [2-AG] and anandamide), and the machinery for their synthesis, degradation, and transport [12,13,14,15,16]. The eCB system plays important roles in synaptic plasticity [9, 17,18,19,20,21] but also has roles in a variety of cellular processes [13, 22,23,24,25,26].
Studies have shown that chronic EtOH exposure transiently decreases the expression and function of CB1 receptors [27,28,29,30,31], but then produces a persistent increase in their expression, particularly in the hippocampus, after alcohol consumption ends [30]. Chronic exposure negatively impacts LTD of synaptic transmission in the hippocampus and other brain structures [9, 32, 33], and in particular, impairs CB1 receptor-dependent LTD [9, 34,35,36], as well as metabotropic glutamate receptor (mGluR) 5-dependent LTD [37]. Despite this evidence, the protracted effects of binge EtOH exposure on the eCB-dependent synaptic plasticity remain largely unknown.
In this study, we investigated the effects of a binge model of alcohol intake on the localization and function of CB1 receptors at the excitatory MPP synapses in the adult DGML of the hippocampus. We focused on these synapses because they are the first to integrate the hippocampal excitatory tri-synaptic circuit involved in learning and memory, and have been shown to be altered by persistent EtOH intake during the adolescence. Our results revealed that chronic EtOH intake during adolescence reduced CB1 receptor expression and impaired eCB-eLTD at the MPP-granule cell synapses. Conversely, inhibition of the monoacylglycerol lipase (MAGL) rescued the recognition memory impairments in these animals.
Materials and methods
Animals
Three-week-old male mice (C57BL/6J; Janvier Labs) were housed in pairs of littermates in standard Plexiglas cages (17 × 14.3 × 36.3 cm) and allowed to habituate to the environment for at least 1 week before experimental procedures were initiated. All animals were maintained at approximately 22 °C with a 12:12 h light:dark cycle (red light on at 9:00 a.m.). Mice had ad libitum access to food throughout all experiments and water except during EtOH access, as noted later. The protocols for animal care and use were approved by the Committee of Ethics for Animal Welfare of the University of the Basque Country (CEEA/M20/2016/073; CEIAB/2016/074) and were in accordance to the European Communities Council Directive of 22nd September 2010 (2010/63/EU) and Spanish regulations (Real Decreto 53/2013, BOE 08-02-2013). Great efforts were made in order to minimize the number and suffering of the animals used.
Drinking in the dark procedure
Adolescent mice (postnatal day (pnd) 32–56) were randomly assigned to either the water or EtOH experimental group. Mice were subjected to a 4-day DID procedure [38], for a total of 4 weeks. Each week, animals were weighed 1 h before lights out on days 1–4. On days 1–4, starting 3 h into the dark cycle, all animals were housed individually in standard Plexiglas cages and were provided with a single bottle of EtOH [20% EtOH (v/v) prepared from EtOH 96% (Alcoholes Aroca S.L., Madrid, Spain)] or tap water for 2 h on days 1–3 and for 4 h on day 4. The EtOH exposure was followed by 3 days abstinence (see Fig. 1a for details of experimental schedule). All animals were given ad libitum access to tap water during the three abstinent days. This schedule was repeated each week for 4 weeks. All animals were pair housed on abstinence and on post-drinking days. To ensure that the effects were the result of voluntary EtOH intake, the amount of EtOH ingested by animals throughout the treatment was measured (see Mathematics and Equations section for more details). Daily drinking amounts over the 4-week DID (Fig. 1b) and the total EtOH intake (g/kg/h) averaged 2.19 ± 0.10 g/kg/h (Fig. 1c). In addition, a blood sample from the lateral tail vein was collected only once 30 min after the last 4-h-EtOH exposure on day 4 of the last week to EtOH access using a capillary tube (Sarstedt, Germany). This blood was subsequently analyzed for EtOH concentration using an EtOH Assay Kit (Sigma-Aldrich) and yielded an average of 62.67 ± 2.67 mg/dl [Fig. 1d, (n = 12) unpaired t-test; ***p < 0.0001]. The correlation between total EtOH intake throughout adolescence period and BEC measured at the end of the EtOH access was also calculated (Fig. 1e). Following the 4 weeks, the animals were in abstinence and all given ad libitum access to tap water until their euthanasia at adulthood (pnd 74–78).
Experimental timeline, voluntary oral EtOH consumption, BEC, and correlation between total EtOH intake and BEC. a EtOH-exposed mice had free EtOH access [20% (v/v)] during 4 weeks in the adolescence (pnd 32–56). Each week, the mice were exposed to 2 or 4 h of free EtOH access, as required. After 2 weeks of withdrawal (i.e. during adulthood), 42 animals (13 per experimental group) were treated with JZL184 or vehicle for five consecutive days (pnd 67–71), and subjected to the NOR test the last 3 days of JZL184 treatment (pnd 69–71). The remaining animals were sacrificed for electrophysiology, anatomy, biochemistry, and molecular techniques in adulthood (pnd 74–78). b Daily EtOH intake during DID [grams of EtOH per kilogram per 2 or 4 h (g/kg/2 or 4 h)]. c Total EtOH intake (g/kg/h) during adolescence (pnd 32–56) and d BEC (mg/dL) of C57BL/6J mice at the last day of EtOH treatment (pnd 56). e Correlation between total EtOH intake throughout adolescence period and BEC measured at the end of the EtOH access. f Left: Stimulating electrode (S) placed in the middle 1/3 of the DML to stimulate MPP. R recording electrode, GC granule cells, MCF mossy cell fibers, LPP lateral perforant path. Right: Time course of LY354740 [1 µM]-induced inhibition of evoked fEPSP after MPP stimulation (filled circles) normalized to baseline. All data are expressed as mean ± SEM. Unpaired t- test; ***p < 0.001
Novel object-recognition test and MAGL inhibitor treatment
During the last days of abstinence, cognitive function was evaluated using the novel object-recognition (NOR) test (see Fig. 1a). This was conducted in a square-shaped open field (40 × 40 × 40 cm) made out of white Plexiglas. Sham and EtOH-treated animals (pnd 69–70) were subjected to a 2-day habituation period, followed by the training and test session on the third day (pnd 71) (modified from ref. [39]). The MAGL inhibitor, JZL184, or the vehicle solution alone was injected intraperitoneally (8 mg/kg) during 4 days before and in the day of the test session (pnd 67–71; see Fig. 1a). Short-term memory was tested 2 h after the training session. A discrimination index was calculated as the difference in exploration time between novel and familiar object divided by the total exploration time with both objects (see Mathematics and Equations section for more details).
Slice preparation and extracellular field recordings
Adult C57BL/6J (pnd 74–78) were anesthetized by inhalation of isoflurane and their brains were rapidly removed and placed in a sucrose-based solution at 4 °C that contained (in mM): 87 NaCl, 75 sucrose, 25 glucose, 7 MgCl2, 2.5 KCl, 0.5 CaCl2, and 1.25 NaH2PO4. Coronal sections (300-μm-thick) were cut with a vibratome (Leica Microsistemas S.L.U.), then were recovered at 32–35 °C and superfused (2 mL/min) in the recording chamber with artificial cerebrospinal fluid (ACSF) containing (in mM): 130 NaCl, 11 glucose, 1.2 MgCl2, 2.5 KCl, 2.4 CaCl2, 1.2 NaH2PO4, and 23 NaHCO3, equilibrated with 95% O2/5% CO2. All experiments were carried out at 32–35 °C. The superfusion medium contained picrotoxin (100 μM) to block GABAA receptors. All drugs were added to their final concentration in the superfusion medium.
For extracellular field recordings, a glass recording pipette was filled with ACSF. The stimulation electrode was placed in the MPP and the recording pipette in the inner 1/3 of the DGML (mossy cell fiber layer) (Fig. 1f). See Supplementary Materials and Methods for details. A low frequency stimulation (LFS, 10 min at 10 Hz) protocol was applied to induce eCB-eLTD of glutamatergic inputs following recording of a steady baseline in the presence of drugs [40, 41].
The fEPSP slope, area, and amplitude were measured (graphs depict area). MPP stimulation was confirmed by the activation of group II mGluRs. Consistent with previous reports [42,43,44], 1 μM of LY354740 strongly reduced MPP-fEPSPs by 53.58 ± 13.16% 10 min after drug application (n = 4, ***p < 0.0001; Fig. 1f). The magnitude of the eCB-eLTD after LFS stimulation was calculated as the percentage change between baseline (averaged excitatory responses for 10 min before LFS) and last 10 min of stable responses, normally at 30 min after the end of the LFS. The slices used for each experimental condition (n) were obtained from at least three mice.
Electron microscopy
Adult C57BL/6J and global CB1 receptor knockout (CB1-KO) mice (n = 3, pnd 76) were deeply anesthetized with ketamine/xylazine (80/10 mg/kg body weight) and transcardially perfused at room temperature (RT, 20–25 °C) with phosphate-buffered saline (PBS, 0.1 M, pH 7.4) and then fixed with 300 ml of 4% formaldehyde (freshly depolymerized from paraformaldehyde), 0.2% picric acid, and 0.1% glutaraldehyde in phosphate buffer (PB) (0.1 M, pH 7.4) prepared at 4 °C. Coronal hippocampal vibrasections were cut at 50 µm and collected in a 0.1 M PB (pH 7.4) at RT. A pre-embedding silver-intensified immunogold method was used for the localization of the CB1 receptor protein [24, 45]. See Supplementary Materials and Methods for details.
Semi-quantification analysis
Receptor density was performed according to the protocol by Puente et al. [45]. See Supplementary Materials and Methods for details.
RNA isolation and qRT-PCR analysis
Total RNA was extracted from the mouse hippocampus (~25–50 mg) by using the Trizol method, as previously described [46, 47]. See Supplementary Materials and Methods for details.
Hippocampal membrane preparation
Western blots of Gαi/o subunits and [35S] GTPγS binding assays were performed using mouse hippocampal membranes (P2 fraction). See Supplementary Materials and Methods for details.
Protein determination by western blot assays
Western blot experiments of Gαi/o subunits were performed as previously described [48] with minor modifications (see Supplementary Materials and Methods).
[35S] GTPγS-binding assays
The [35S] GTPγS-binding assays were performed following the procedure described elsewhere [49] (see Supplementary Materials and Methods).
Measurement of hippocampal endogenous 2-AG and arachidonic acid levels by liquid chromatography tandem mass spectrometry (LC/MS)
The determination of the endogenous 2-AG and arachidonic acid (AA) levels was carried out as described by Schulte et al. [50] with minor modifications [51] (see Supplementary Materials and Methods).
Experimental design and statistical analysis
All values are given as mean ± standard error of the mean (SEM) with p values and sample size (n). Shapiro–Wilk and Kolmogorov–Smirnov tests were used to confirm normality of the data. Statistical significance between groups [sham versus (vs) EtOH-treated mice] was tested using parametric or non-parametric two-tailed Student’s t-test, as required. Data obtained from NOR test were analyzed using a one-way analysis of variance (ANOVA). The significance level was set at p < 0.05 for all comparisons. All statistical tests were performed with GraphPad Prism (GraphPad Prism 5).
Drugs and chemicals
CP 55.940, Win 55,212-2 (Win-2), AM251, URB 597, JZL184, AM404, and picrotoxin were obtained from Tocris BioScience (Bristol, UK). JZL184 was administered in a volume of 10 mL/kg, dissolved in 15% dimethyl sulfoxide (DMSO; Sigma-Aldrich):4.25% polyethylene glycol 400 (Sigma-Aldrich):4.25% Tween-80 (Sigma-Aldrich):76.5% saline.
2-AG and AA and their deuterated analogs, 2-AG-d5 and AA-d8, used for LC/MS determinations were obtained from Cayman Chemicals.
Results
Adolescent EtOH intake impairs adult CB1 receptor-mediated excitatory transmission and eCB-eLTD at MPP-granule cell synapses
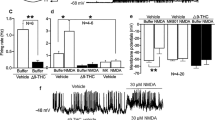
The input–output relationships between fEPSPs slope relative to stimulus intensity revealed a significant decrease in the slope of the fEPSP for EtOH-exposed animals (*p < 0.05 vs sham) (Fig. 2a, A′) suggesting that adolescent EtOH consumption affects basal synaptic transmission in the adult. This decrease could be due to an increase in eCB-signaling during or after EtOH consumption. To test if this decrease in synaptic efficacy was due to the activation of CB1 receptors by a different type of eCBs present in sham vs EtOH-treated mice, we used the CB1 receptor antagonist AM251. We found that AM251 [4 µM] had no significant different effect on the fEPSP amplitude of sham and EtOH-treated mice (Fig. 2b). On the other hand, the CB1 receptor-induced suppression of the fEPSP normally observed at MPP-granule cell synapses in sham mice [41] was not observed in EtOH-treated mice after withdrawal [Fig. 2c, d: CP 55.940, 10 µM: (n = 8) 110.3 ± 7.25 % of fEPSP; Mann–Whitney U test; p = 0.08 vs baseline and Win-2, 5 µM: (n = 7) 104.7 ± 7.08% of fEPSP Mann–Whitney U test; p = 0.9273 vs baseline]. Furthermore, the eCB-eLTD elicited by MPP stimulation (10 Hz/10 min) was abolished by the CB1 receptor antagonist, AM251 [(4 µM: (n = 4) 103.9 ± 9.71% of fEPSP; Mann–Whitney U test; p = 1.00 vs baseline] as previously described [41] (Fig. 2e, f). Remarkably, eCB-eLTD was absent in EtOH-treated mice [Fig. 2e, f (n = 16) 103.1 ± 2.77% of fEPSP; Mann–Whitney U test; p = 0.1704 vs baseline] that could relate to the decreased fEPSPs observed in EtOH (Fig. 2a, A′). Altogether, these findings demonstrate that chronic exposure to EtOH during adolescence has long-term impacts on the CB1-receptor-mediated excitatory synaptic transmission and eCB-eLTD at the MPP-granule cell synapses in the mature brain.
EtOH intake during adolescence impairs adult CB1 receptor-mediated excitatory transmission and eCB-eLTD at MPP synapses. a Input–output curves where mean fEPSP areas (mv/ms) are plotted against the stimulation intensities in hippocampal slices of sham (whitecircles) and EtOH mice (black circles). Mann–Whitney U test; *p < 0.05 vs sham. b The CB1 antagonist AM251 (4 µM, white circles) does not change the area of the fEPSP in sham or EtOH-treated mice (black circles). c CP 55.940 (10 µM, white circles) reduces the fEPSP in sham but not in EtOH-treated mice (black circles). d Summary bar graph of the experiments performed: baseline, sham + CP 55.940 [10 µM], EtOH + CP 55.940 [10 µM], EtOH + Win-2 [5 µM]. Numbers in the bars are individual transmission experiments. Data are expressed as mean ± SEM. Mann–Whitney U test, p > 0.05; *p ≤ 0.05; ***p < 0.001 vs baseline. e LFS (10 min, 10 Hz) triggers eCB-eLTD in sham (white circles) but not in EtOH group (black circles). Data are expressed as mean ± SEM. Mann–Whitney U test, p > 0.05; *p ≤ 0.05 vs baseline. f Summary bar graph of eCB-eLTD experiments performed: sham, sham + AM251 and EtOH. Numbers in the bars are individual experiments. Data are expressed as mean ± SEM. Mann–Whitney U test *p ≤ 0.05
Adolescent EtOH intake induces significant changes in gene and protein expression of the eCB system in the mature hippocampus
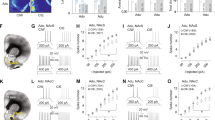
The expression of both the CB1 receptor gene, CNR1 [Fig. 3a (n = 16) unpaired t-test; **p = 0.005] and its protein [Fig. 3a (n = 8) unpaired t-test; *p = 0.035] [Figs. S1 and S2] was significantly reduced after EtOH exposure during adolescence followed by 2 weeks of EtOH withdrawal. In contrast, a significant increase in the MAGL gene, MGLL [Fig. 3a (n = 16) unpaired t-test; **p = 0.003] and its protein [Fig. 3a (n = 8) unpaired t-test; **p = 0.0012] [Figs. S1 and S2] relative to sham was detected. In addition, mGluR5 mRNA was significantly decreased upon adolescent exposure to EtOH [Fig. 3a (n = 8) unpaired t-test; *p = 0.03] but no significant changes were observed in protein levels [Fig. 3a (n = 5) Mann–Whitney U test; p = 0.31]. Furthermore, the DAGL-α and DAGL-β genes encoding for the DAGL-α and DAGL-β enzymes [Fig. 3a (n = 16) unpaired t-test; p = 0.76, and p = 0.44, respectively], and the NAPE-PLD and FAAH mRNAs [Fig. 3a (n = 16) unpaired t-test; p = 0.19; p = 0.053, respectively] did not show any significant change as a result of the adolescent EtOH exposure.
Molecular changes in the endocannabinoid and glutamatergic systems after EtOH intake during adolescence. a Relative CNR1 mRNA and CB1 receptor protein (mRNA: unpaired t-test; **p < 0.01; protein: unpaired t-test; *p < 0.05), relative MGLL mRNA and MAGL protein (mRNA: unpaired t-test; **p < 0.01; protein: unpaired t-test; **p < 0.01), relative GRM5 mRNA and mGluR5 protein (mRNA: unpaired t-test; *p < 0.05; protein: Mann–Whitney U test; p > 0.05), relative mRNA levels of DAGL-α (unpaired t-test; p > 0.05) and DAGL-β (unpaired t-test; p > 0.05), and relative FAAH mRNA and NAPE-PLD mRNA in adult hippocampus of sham and EtOH-treated mice during adolescence (unpaired t-test; p > 0.05); numbers in the bars are individual experiments. b 2-AG and AA levels in individual homogenates from hippocampal brain samples (Mann–Whitney U test; p > 0.05; **p = 0.008). c Computer-assisted curve fitting of CP 55.940-stimulated [35S] GTPγS binding in hippocampal membranes from sham and EtOH-treated mice. Concentration–response curves were constructed using mean values ± SEM from four different experiments performed in duplicate. Mann–Whitney U test; p > 0.05, ns; *p < 0.05; **p < 0.01. Bar graphs in the inset depict the relative percentage of [35S] GTPγS basal binding levels in sham and EtOH. Data in the inset are mean ± SEM. Mann–Whitney U test. *p < 0.05. d Values of CP 55.940-stimulated [35S] GTPγS to hippocampal brain membranes from sham and EtOH-treated mice. Data values are mean ± SEM of four different experiments performed in duplicate. Significance of difference from the corresponding values in control counterparts (Mann–Whitney U test; p > 0.05; *p < 0.05; **p < 0.01) is shown. e Relative expression levels of Gαo, Gαi1, Gαi2, and Gαi3 subunits in hippocampal membrane samples from sham and EtOH. The number of samples analyzed is in parentheses on the lower aspect of each label. t-test with Welch’s correction; p > 0.05; *p < 0.05. All data are expressed as mean ± SEM
Adolescent exposure to EtOH alters endogenous AA levels and CP 55.940-stimulated [35S] GTPγS binding in adulthood
The endogenous 2-AG and AA levels were assessed by LC/MS. In the sham hippocampus, basal 2-AG was 6.92 ± 0.42 nmol/g and in the EtOH group it was 6.65 ± 0.84 nmol/g [Fig. 3b (n = 5) Mann–Whitney U test; p = 0.55]. In contrast, AA levels in the sham were significantly lower (21.18 ± 1.79 nmol/g) than in the hippocampus of the EtOH-treated mice (76.30 ± 4.61 nmol/g) [Fig. 3b (n = 5) Mann–Whitney U test; **p = 0.008].
[35S] GTPγS-binding assays were performed with the CB1 receptor agonist CP 55.940 in hippocampal membranes obtained from both sham and EtOH-treated mice. As shown in Fig. 3c, CP 55.940 was able to stimulate [35S] GTPγS binding in a concentration-dependent manner in both cases without significant differences in efficacy (Emax) [Fig. 3d (n = 4) Mann–Whitney U test; p = 0.25]. However, the potency of CP 55.940-stimulated [35S] GTPγS binding was three- to four-fold higher in sham than in EtOH-treated mice (EC50) [Fig. 3d (n = 4) 45.7 ± 13.2 nM and 148.5 ± 24.1 nM, respectively; Mann–Whitney U test; **p = 0.008]. Furthermore, a significant reduction (~18%) in [35S] GTPγS basal binding was observed in hippocampal membranes of EtOH-treated mice [Fig. 3d; inset of the Fig. 3c (n = 4) Mann–Whitney U test; *p = 0.02].
In order to evaluate whether the changes observed in [35S] GTPγS-binding assays were related to any alteration in G-protein expression, the relative expression levels of different Gαi/o subunits were determined by western blotting. No differences in the Gαo, Gαi1, and Gαi3 subunits were found between sham and EtOH-treated mice [Fig. 3e (n = 2–3) t-test with Welch’s correction; p = 0.09]. However, the Gαi2 subunit showed a significant (16%) decrease in hippocampal membranes of EtOH-treated mice relative to sham [Fig. 3e (n = 3) t-test with Welch’s correction; *p = 0.029].
Subcellular localization of CB1 receptors in the adult dentate MPP termination zone after chronic EtOH exposure during adolescence
CB1 receptor immunogold particles in the middle 1/3 of the DGML of sham and EtOH-treated mice were mainly localized to inhibitory and excitatory axon terminals forming synapses with dendrites and dendritic spines, respectively (Fig. 4a–d). The CB1 receptor immunolabeling was absent in the global CB1-KO mice (Fig. 4e) demonstrating the specificity of the anti-CB1 receptor antibody used.
Ultrastructural localization of CB1 receptors in the middle 1/3 of the DGML. a–d CB1 receptor immunogold labeling (black arrows) is observed on both excitatory terminals (ter) forming asymmetric synapses (white arrowheads) with dendritic spines (sp) and on inhibitory preterminals (preter) in sham and EtOH-treated mice. Scale bars: 0.5 µm. e Only residual CB1 receptor immunolabeling was detected in CB1-KO. Scale bars: 0.5 µm. f Proportion of CB1 receptor labeling in different compartments normalized to the total CB1 receptor signal in sham and EtOH-treated mice. g Percentage of CB1 receptor-immunopositive excitatory synaptic terminals in sham, EtOH, and CB1-KO. The number of synaptic terminals analyzed is in parentheses on the top of each column. h CB1 receptor density (particles/µm) in CB1 receptor positive excitatory terminals in sham and EtOH-treated mice. The number of synaptic terminals is in parentheses on the top of each column. Data are expressed as mean ± SEM. Unpaired t-test or Mann–Whitney U test, p > 0.05; *p < 0.05; ***p < 0.001
To determine whether adolescent EtOH intake caused a global change in CB1 receptor expression in the mature hippocampus, the CB1 receptor immunoparticle distribution (% of CB1 immunoparticles distributed in different compartments taken from the total CB1 particles counted in the middle 1/3 DGML) was compared between sham and EtOH-treated mice. The values in sham mice were: excitatory terminals (14.56 ± 2.45%), inhibitory terminals (46.08 ± 4.96%), mitochondria (11.65 ± 1.31%), dendrites (10.69 ± 1.35%), other membranes (17.02 ± 2.26%). In EtOH-treated mice: excitatory terminals (9.52 ± 0.93%), inhibitory terminals (49.70 ± 5.08%), mitochondria (11.80 ± 1.38%), dendrites (12.84 ± 1.54%), other membranes (17.19 ± 2.08%) [Fig. 4f (n = 3) Mann–Whitney U test; p = 1.00]. Furthermore, the percentage of CB1 receptor-labeled excitatory terminals was significantly reduced after EtOH exposure [Fig. 4g (n = 3) 17.78 ± 1.95% in EtOH vs 26.98 ± 3.15% in sham; unpaired t-test; *p = 0.02]. Excitatory terminals with residual CB1 receptor immunolabeling were negligible in the global CB1-KO mice (Fig. 4g, 2.32 ± 0.92%. Mann–Whitney U test; ***p < 0.0001). No statistical differences were found in CB1 receptor immunoparticle density (particles per µm) between excitatory boutons of sham (0.63 ± 0.05) and EtOH-treated mice (0.58 ± 0.03) [Fig. 3h (n = 3) Mann–Whitney U test; p = 0.22).
Enhancement of 2-AG signaling normalizes eCB-eLTD in EtOH-treated mice
Bath application of JZL184 [50 µM, >1 h] rescued eCB-eLTD in EtOH-treated mice [Fig. 5a, c (n = 14) 84.98 ± 4.60% of fEPSP; Mann–Whitney U test; *p = 0.012 vs baseline; **p = 0.004 vs EtOH-treated mice], indicating that the endogenous 2-AG tone is affecting eCB-eLTD at MPP synapses following EtOH exposure. Furthermore, the eLTD restored by JZL184 was CB1-receptor dependent since AM251 (4 µM) blocked eCB-eLTD [Fig. 5c (n = 8) 103.6 ± 6.37% of fEPSP; Mann–Whitney U test; p = 0.88 vs EtOH-treated mice). However, URB 597 (2 µM, 20 min) did not produce any changes in the evoked fEPSP [Fig. 5b, c (n = 5) 102.9 ± 3.95% of fEPSP; Mann–Whitney test; p = 0.90 vs EtOH-treated mice]. Also, the anandamide transporter inhibitor, AM404 (30 µM) did not elicit eCB-eLTD [Fig. 5c (n = 5) 114.8 ± 4.23%; Mann–Whitney U test, p = 0.22 vs baseline; p = 0.06 vs EtOH-treated mice]. These findings reveal that the pharmacological blockade of 2-AG degradation rescues eCB-eLTD in adult MPP-granule cell synapses after adolescent EtOH exposure.
Enhancement of 2-AG signaling normalizes eCB-eLTD and reverses adult cognitive impairment in EtOH-treated mice. a Time course plot of average fEPSP areas upon application of the LFS (10 min at 10 Hz) in sham (white circles), EtOH-treated (black circles), and EtOH-treated mice with MAGL inhibitor (JZL184, 50 µM, >1 h, gray circles). JZL184 recovers eCB-eLTD in EtOH-treated animals. Data are expressed as mean ± SEM. Mann–Whitney test, p > 0.05; *p < 0.05 vs baseline. b Time course plot of average fEPSP areas upon application of the LFS protocol in sham (white circles), EtOH-treated (black circles), and EtOH-treated mice with the FAAH inhibitor URB 597 (2 µM, >20 min, gray circles). URB 597 has no effect on the loss eCB-eLTD after EtOH exposure. Data are expressed as mean ± SEM. Mann–Whitney U test, p > 0.05; **p < 0.01 vs baseline. c Summary bar graph of the experiments performed: sham, EtOH, EtOH + JZL184 [50 µM, >1 h], EtOH+ (JZL184 + AM251) cocktail [JZL184: 50 µM, >1 h; AM251: 4 µM, >30 min], EtOH + URB 597 [2 µM, >20 min] and EtOH + AM404 [30 µM]. Numbers in the bars are individual experiments. All data are expressed as mean ± SEM Mann–Whitney U test, p > 0.05; **p < 0.01 vs sham. d Discrimination index of each experimental group in 10 min testing session of the NOR test. e Exploration time (seconds) of objects in the 10 min test session. Each bar represents the mean ± SEM (n = 13). One-way ANOVA and Bonferroni’s Multiple Comparison Test if required; p > 0.05; ***p < 0.001
JZL184 reverses cognitive impairment induced by EtOH treatment
In the NOR test, adult mice treated with EtOH during adolescence showed a much lower short-term memory discrimination index than the sham [Fig. 5d (n = 13) Bonferroni’s Multiple comparison test; ***p < 0.001], as shown previously by Vetreno and Crews [10]. However, systemic JZL184 administration (8 mg/kg ip) vastly improved the discrimination index and restored memory consolidation to sham values [Fig. 5d (n = 13) Bonferroni’s multiple comparison test; ***p < 0.001]. No differences in the total exploration time were observed among the experimental groups [Fig. 5e (n = 13) one-way ANOVA, p = 0.60].
Discussion
The novelty of our findings resides in that EtOh intake during adolescence (binge drinking model) causes a severe and selective long-lasting reduction in CB1 receptors localized on the excitatory MPP synaptic terminals (but not on other subcellular compartments in the middle 1/3 of the DGML) of the adult mouse hippocampus that associates with loss of eCB-eLTD at the MPP synapses and recognition memory impairment. Interestingly, both synaptic plasticity and memory can be rescued by increasing the endogenous 2-AG. Alterations in eCB metabolism and signaling pathways during critical periods of brain development cause long-lasting behavioral abnormalities that can be detected into adulthood [52, 53]. EtOH consumption alters eCB-dependent synaptic plasticity leading to long-term cognitive impairments [9, 38, 54,55,56,57,58]. Reciprocally, the eCBs play a pivotal role in the EtOH drinking behavior and in the development of alcoholism [57, 59] but it prevents, at the same time, the potentiation of GABA release and glutamatergic transmission elicited by EtOH [60,61,62]. The findings of our study demonstrated that chronic EtOH intake during adolescence severely disrupts CB1 receptor-mediated excitatory transmission and long-term plasticity in adult MPP-granule cell synapses resulting in memory impairment. This is particularly intriguing that the effects of chronic EtOH intake during adolescence are strikingly long lasting. Moreover, the amount consumed and BEC achieved by the mice are fairly modest, indicating that large amounts of EtOH are not necessary in order for lasting effects on the eCB system to be achieved.
Long-term effects of EtOH intake during adolescence
The disruption of the adult CB1 receptor-mediated excitatory transmission and eCB-eLTD after adolescent EtOH intake is supported by previous findings [35, 63,64,65]. Furthermore, the absence of eCB-eLTD in our study was associated with a defect in recognition memory in adulthood. A reduction in neurogenesis [10, 66], an increase in neuroinflammation [67, 68], or an increase in neurodegeneration [69] might be playing a role in the memory deficits observed. However, it turns out that the recognition memory impairment shown in the model of binge drinking applied in this study correlates with a cannabinoid signaling disturbance, as the loss of excitatory synaptic plasticity and NOR deficits were reversible by MAGL inhibition. These results are consistent with previous studies supporting a role of 2-AG in NOR [70,71,72]. Moreover, these changes were correlated with a significant decrease in the relative CB1 receptor mRNA and protein, as shown before [27, 30, 73], as well as by a significant decrease in the amount of CB1 receptor immunoparticles in excitatory terminals and astroglia in the CA1 hippocampus [38]. In the present study, CB1 receptor immunolabeling decreased by 34% in excitatory terminals and the proportion of CB1 receptor immunopositive excitatory boutons decreased by 35% in the DGML middle 1/3 of EtOH-treated versus sham; no changes in the CB1 receptor expression were detected in other subcellular compartments. Hence, the CB1 receptor reduction in excitatory terminals could account for at least part of the deficits in the adult eCB-eLTD after adolescent EtOH intake.
CB1 receptors located on glutamatergic synapses are tightly coupled to G-protein signaling [74]; thus, CB1 receptor signaling might also be affected by EtOH intake in the adolescence. We found a significant reduction in CP 55.940 potency for stimulating [35S] GTPγS binding and [35S] GTPγS basal binding that agrees with the decrease in CB1 receptor binding [27, 29] and G-protein cycling after EtOH [75]. Furthermore, we also detected a specific reduction in the Gαi2 subunit that might be responsible for the observed reduction in [35S] GTPγS basal binding and the impairment in CB1 receptor signaling, which may be related to the absence of eCB-eLTD and deficits in the NOR test in the EtOH-treated mice. In fact, a lack of Gαi2 subunit leads to abnormalities in learning efficiency, sociability and social recognition [76]. As a compensatory mechanism, there was an increase in MAGL in our EtOH model, as shown by others [53], but no changes in the 2-AG biosynthetic enzyme mRNA expression were detected. Consequently, 2-AG levels were expected to decrease in animals exposed to EtOH and, curiously, there were no changes in 2-AG levels after withdrawal. However, a substantial increase in AA was noticed, suggesting that 2-AG increased during or after EtOH exposure [77] that could eventually be normalized by more 2-AG degradation through the increase in MAGL activity. A hypothetical fluctuation in 2-AG levels might be explaining the decrease in fEPSP slope in EtOH-exposed animals. It would be very interesting to test in the future how repeated JZL184-treatment impacts on the EtOH fEPSP. The increase in AA levels in EtOH might also be pointing to changes in anandamide. However, we were not able to observe any variation in the expression of NAPE-PLD and FAAH mRNAs in EtOH-exposed mice suggesting that anandamide was not being primarily altered in our model of adolescent binge drinking. Furthermore, the anandamide increase by the FAAH inhibitor URB597 was unable to rescue LTD in EtOH-treated mice.
The adolescent EtOH impairs NOR memory after cessation of consumption, as previously shown [78,79,80], which could be attributable to its effects on developing hippocampal, parahippocampal, and neocortical structures leading to a deficit in recognition memory formation [81]. Interestingly, MAGL inhibition was able to overcome the functional and behavioral disturbances induced by EtOH, most likely due to the increase in 2-AG. Actually, pharmacological or genetic ablation of MAGL have been shown to enhance long-term synaptic plasticity, improve cognitive performance through CB1 receptor-mediated mechanisms, suppress neuroinflammation, and prevent neurodegeneration after harmful insults [82, 83]. Thus, upon agonist (2-AG)-induced stimulation of Gαi/o subunits, inhibition of MAGL could overcome the loss of CB1 receptors in glutamatergic terminals due to the high coupling efficiency of this CB1 receptor population [74], leading to functional (eCB-eLTD) and behavioral (recognition memory) recovery in adult mice after EtOH treatment during adolescence. MAGL inhibition in vivo may primarily act by suppressing GABAA receptor-mediated inhibition; therefore, CB1 receptors localized in GABAergic terminals might also be contributing indirectly to the eCB-eLTD recovery in EtOH-treated mice.
Taken together, the increase in MAGL activity, the decrease in CB1 receptors in excitatory terminals, and their loss of efficacy could be underlying the absence of eCB-eLTD at the MPP-granule cell synapses and the memory impairment observed in mature mice after EtOH exposure during adolescence. The present results offer future investigations oriented to the search for new therapies to minimize the potential consequences in adulthood of binge EtOH intake during early periods of life.
Funding and Disclosure
This work was supported by ISCIII (“RD16/0017/0012” to PG), co-funded by ERDF/ESF, “Investing in your future”; The Basque Government (IT1230-19 to PG]; MINECO/FEDER, UE (SAF2015-65034-R to P.G.); University of the Basque Country (UPV/EHU PPG17/70 to J.S.); ISCIII (“RD16/0017/001” to F.R.F.), co-funded by ERDF/ESF, “Investing in your future”; Proyectos de investigación en Salud (PI16/01698 to F.R.F.); Ph.D. contract from MINECO (BES-2013-065057 to S.P.); Vanier Canada Graduate Scholarship (NSERC to C.J.F.). B. Christie and P. Nahirney are supported by grants from the Canadian Institutes of Health Research. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Kyzar EJ, Floreani C, Teppen TL, Pandey SC. Adolescent alcohol exposure: burden of epigenetic reprogramming, synaptic remodeling, and adult psychopathology. Front Neurosci. 2016;10:222.
Cservenka A, Brumback T. The burden of binge and heavy drinking on the brain: effects on adolescent and young adult neural structure and function. Front Psychol. 2017;8:1111.
Merrill JE, Carey KB. Drinking over the lifespan: focus on college ages. Alcohol Res. 2016;38:103–14.
Pava MJ, Woodward JJ. A review of the interactions between alcohol and the endocannabinoid system: implications for alcohol dependence and future directions for research. Alcohol. 2012;46:185–204.
Lovinger DM, Roberto M. Synaptic effects induced by alcohol. Curr Top Behav Neurosci. 2013;13:31–86.
Keshavan MS, Giedd J, Lau JYF, Lewis DA, Paus T. Changes in the adolescent brain and the pathophysiology of psychotic disorders. Lancet Psychiatry. 2014;1:549–58.
Abrahao KP, Salinas AG, Lovinger DM. Alcohol and the brain: neuronal molecular targets, synapses, and circuits. Neuron. 2017;96:1223–38.
Lovinger DM, Alvarez VA. Alcohol and basal ganglia circuitry: animal models. Neuropharmacology. 2017;122:46–55.
Lovinger DM, Abrahao KP. Synaptic plasticity mechanisms common to learning and alcohol use disorder. Learn Mem. 2018;25:425–34.
Vetreno RP, Crews FT. Binge ethanol exposure during adolescence leads to a persistent loss of neurogenesis in the dorsal and ventral hippocampus that is associated with impaired adult cognitive functioning. Front Neurosci. 2015;9:1–12.
Meyer HC, Lee FS, Gee DG. The role of the endocannabinoid system and genetic variation in adolescent brain development. Neuropsychopharmacology. 2017;43:21.
Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci. 2003;4:873–84.
Katona I, Freund TF. Multiple functions of endocannabinoid signaling in the brain. Annu Rev Neurosci. 2012;35:529–58.
Lutz B, Marsicano G, Maldonado R, Hillard CJ. The endocannabinoid system in guarding against fear, anxiety and stress. Nat Rev Neurosci. 2015;16:705–18.
Pertwee RG. Endocannabinoids and their pharmacological actions. Handb Exp Pharmacol. 2015;231:1–37.
Iannotti FA, Di Marzo V, Petrosino S. Endocannabinoids and endocannabinoid-related mediators: targets, metabolism and role in neurological disorders. Prog Lipid Res. 2016;62:107–28.
Castillo PE, Younts TJ, Chávez AE, Hashimotodani Y. Endocannabinoid signaling and synaptic function. Neuron. 2012;76:70–81.
Chevaleyre V, Castillo PE. Endocannabinoid-mediated metaplasticity in the hippocampus. Neuron. 2004;43:871–81.
Ohno-Shosaku T, Kano M. Endocannabinoid-mediated retrograde modulation of synaptic transmission. Curr Opin Neurobiol. 2014;29:1–8.
Monday HR, Castillo PE. Closing the gap: long-term presynaptic plasticity in brain function and disease. Curr Opin Neurobiol. 2017;45:106–12.
Monday HR, Younts TJ, Castillo PE. Long-term plasticity of neurotransmitter release: emerging mechanisms and contributions to brain function and disease. Annu Rev Neurosci. 2018;41:299–322.
Lu H-C, Mackie K. An introduction to the endogenous cannabinoid system. Biol Psychiatry. 2016;79:516–25.
Araque A, Castillo PE, Manzoni OJ, Tonini R. Synaptic functions of endocannabinoid signaling in health and disease. Neuropharmacology. 2017;124:13–24.
Gutiérrez-Rodríguez A, Puente N, Elezgarai I, Ruehle S, Lutz B, Reguero L. et al. Anatomical characterization of the cannabinoid CB1 receptor in cell-type-specific mutant mouse rescue models. J Comp Neurol. 2017;525:302–18.
Gutiérrez-Rodríguez A, Bonilla-Del Río I, Puente N, Gómez-Urquijo SM, Fontaine CJ, Egaña-Huguet J. et al. Localization of the cannabinoid type-1 receptor in subcellular astrocyte compartments of mutant mouse hippocampus. Glia. 2018;66:1417–31.
Busquets-Garcia A, Oliveira da Cruz JF, Terral G, Zottola ACP, Soria-Gómez E, Contini A. et al. Hippocampal CB1 receptors control incidental associations. Neuron. 2018;99:1247–59.e7.
Basavarajappa BS, Cooper TB, Hungund BL. Chronic ethanol administration down-regulates cannabinoid receptors in mouse brain synaptic plasma membrane. Brain Res. 1998;793:212–8.
Ortiz S, Oliva JM, Pérez-Rial S, Palomo T, Manzanares J. Chronic ethanol consumption regulates cannabinoid CB1 receptor gene expression in selected regions of rat brain. Alcohol Alcohol. 2004;39:88–92.
Vinod KY, Yalamanchili R, Xie S, Cooper TB, Hungund BL. Effect of chronic ethanol exposure and its withdrawal on the endocannabinoid system. Neurochem Int. 2006;49:619–25.
Mitrirattanakul S, López-Valdés HE, Liang J, Matsuka Y, Mackie K, Faull KF. et al. Bidirectional alterations of hippocampal cannabinoid 1 receptors and their endogenous ligands in a rat model of alcohol withdrawal and dependence. Alcohol Clin Exp Res. 2007;31:855–67.
Pava MJ, Woodward JJ. Chronic ethanol alters network activity and endocannabinoid signaling in the prefrontal cortex. Front Integr Neurosci. 2014;8:58
Thinschmidt JS, Walker DW, King MA. Chronic ethanol treatment reduces the magnitude of hippocampal LTD in the adult rat. Synapse. 2003;48:189–97.
Coune F, Silvestre de Ferron B, González-Marín MC, Antol J, Naassila M, Pierrefiche O. Resistance to ethanol sensitization is associated with a loss of synaptic plasticity in the hippocampus. Synapse. 2017;71:e21899.
Xia JX, Li J, Zhou R, Zhang XH, Ge YB, Ru Yuan X. alterations of rat corticostriatal synaptic plasticity after chronic ethanol exposure and withdrawal. Alcohol Clin Exp Res. 2006;30:819–24.
Adermark L, Jonsson S, Ericson M, Söderpalm B. Intermittent ethanol consumption depresses endocannabinoid-signaling in the dorsolateral striatum of rat. Neuropharmacology. 2011;61:1160–5.
DePoy L, Daut R, Brigman JL, MacPherson K, Crowley N, Gunduz-Cinar O. et al. Chronic alcohol produces neuroadaptations to prime dorsal striatal learning. Proc Natl Acad Sci. 2013;110:14783–8.
Wills TA, Baucum AJ, Holleran KM, Chen Y, Pasek JG, Delpire E. et al. Chronic intermittent alcohol disrupts the GluN2B-associated proteome and specifically regulates group I mGlu receptor-dependent long-term depression. Addict Biol. 2017;22:275–90.
Bonilla-Del Rίo I, Puente N, Peñasco S, Rico I, Gutiérrez-Rodrίguez A, Elezgarai I. et al. Adolescent ethanol intake alters cannabinoid type-1 receptor localization in astrocytes of the adult mouse hippocampus. Addict Biol. 2019;24:182–92.
Huang TN, Chuang HC, Chou WH, Chen CY, Wang HF, Chou SJ. et al. Tbr1 haploinsufficiency impairs amygdalar axonal projections and results in cognitive abnormality. Nat Neurosci. 2014;17:240–7.
Puente N, Cui Y, Lassalle O, Lafourcade M, Georges F, Venance L. et al. Polymodal activation of the endocannabinoid system in the extended amygdala. Nat Neurosci. 2011;14:1542–7.
Peñasco S, Rico-Barrio I, Puente N, Gómez-Urquijo SM, Fontaine CJ, Egaña-Huguet J. et al. Endocannabinoid long-term depression revealed at medial perforant path excitatory synapses in the dentate gyrus. Neuropharmacology. 2019;153:32–40.
Macek TA, Winder DG, Gereau RW, Ladd CO, Conn PJ. Differential involvement of group II and group III mGluRs as autoreceptors at lateral and medial perforant path synapses. J Neurophysiol. 1996;76:3798–806.
Chiu CQ, Castillo PE. Input-specific plasticity at excitatory synapses mediated by endocannabinoids in the dentate gyrus. Neuropharmacology. 2008;54:68–78.
Chávez AE, Chiu CQ, Castillo PE. TRPV1 activation by endogenous anandamide tirggers postsynaptic LTD in dentate gyrus. Nat Neurosci. 2010;13:1511–8.
Puente N, Bonilla-Del Río I, Achicallende S, Nahirney P, Grandes P. High-resolution immunoelectron microscopy techniques for revealing distinct subcellular type 1 cannabinoid receptor domains in brain. Bio-protocol. 2019;9:1–18.
Serrano A, Rivera P, Pavon FJ, Decara J, Suárez J, Rodriguez de Fonseca F. et al. Differential effects of single versus repeated alcohol withdrawal on the expression of endocannabinoid system-related genes in the rat amygdala. Alcohol Clin Exp Res. 2012;36:984–94.
Blanco E, Galeano P, Palomino A, Pavón FJ, Rivera P, Serrano A. et al. Cocaine-induced behavioral sensitization decreases the expression of endocannabinoid signaling-related proteins in the mouse hippocampus. Eur Neuropsychopharmacol. 2016;26:477–92.
Montaña M, García del Caño G, López de Jesús M, González-Burguera I, Echeazarra L, Barrondo S. et al. Cellular neurochemical characterization and subcellular localization of phospholipase C β1 in rat brain. Neuroscience. 2012;222:239–68.
Barrondo S, Sallés J. Allosteric modulation of 5-HT1A receptors by zinc: Binding studies. Neuropharmacology. 2009;56:455–62.
Schulte K, Steingrüber N, Jergas B, Redmer A, Kurz CM, Buchalla R. et al. Cannabinoid CB1 receptor activation, pharmacological blockade, or genetic ablation affects the function of the muscarinic auto- and heteroreceptor. Naunyn Schmiedebergs Arch Pharmacol. 2012;385:385–96.
García del Caño G, Aretxabala X, González-Burguera I, Montaña M, López de Jesús M, Barrondo S. et al. Nuclear diacylglycerol lipase-α in rat brain cortical neurons: evidence of 2-arachidonoylglycerol production in concert with phospholipase C-β activity. J Neurochem. 2015;132:489–503.
Subbanna S, Shivakumar M, Psychoyos D, Xie S, Basavarajappa BS. Anandamide-CB1 receptor signaling contributes to postnatal ethanol-induced neonatal neurodegeneration, adult synaptic, and memory deficits. J Neurosci. 2013;33:6350–66.
Subbanna S, Psychoyos D, Xie S, Basavarajappa BS. Postnatal ethanol exposure alters of 2-arachidonoilglycerol-metabolizing enzymes and pharmacological inhibition of monoacylglycerol does not cause neurodegeneration in neonatal mice. J Neurochem. 2015;36:1011–4.
DePoy L, Daut R, Wright T, Camp M, Crowley N, Noronha B. et al. Chronic alcohol alters rewarded behaviors and striatal plasticity. Addict Biol. 2015;20:345–8.
Crews FT, Vetreno RP, Broadwater MA, Robinson DL. Adolescent alcohol exposure persistently impacts adult neurobiology and behavior. Pharm Rev. 2016;68:1074–109.
Nimitvilai S, Lopez MF, Mulholland PJ, Woodward JJ. Chronic intermittent ethanol exposure enhances the excitability and synaptic plasticity of lateral orbitofrontal cortex neurons and induces a tolerance to the acute inhibitory actions of ethanol. Neuropsychopharmacology. 2016;41:1112–27.
Lovinger DM. Presynaptic ethanol actions: potential roles in ethanol seeking. Berlin, Heidelberg: Springer Berlin Heidelberg; 2017. p. 1–26.
Marco EM, Peñasco S, Hernández M-D, Gil A, Borcel E, Moya M. et al. Long-term effects of intermittent adolescent alcohol exposure in male and female rats. Front Behav Neurosci. 2017;11:1–13.
Basavarajappa BS, Hungund BL. Neuromodulatory role of the endocannabinoid signaling system in alcoholism: an overview. Prostaglandins Leukot Essent Fatty Acids. 2002;66:287–99.
Basavarajappa BS, Ninan I, Arancio O. Acute ethanol suppresses glutamatergic neurotransmission through endocannabinoids in hippocampal neurons. J Neurochem. 2008;107:1001–13.
Roberto M, Cruz M, Bajo M, Siggins GR, Parsons LH, Schweitzer P. The endocannabinoid system tonically regulates inhibitory transmission and depresses the effect of ethanol in central amygdala. Neuropsychopharmacology. 2010;35:1962–72.
Talani, L. Interactions between ethanol and the endocannabinoid system at GABAergic synapses on basolateral amygdala principal neurons. Alcohol. 2015;4:139–48.
Guerri C, Pascual M. Mechanisms involved in the neurotoxic, cognitive, and neurobehavioral effects of alcohol consumption during adolescence. Alcohol. 2010;44:15–26.
Renteria R, Jeanes ZM, Morrisett RA. Ethanol attenuation of long-term depression in the nucleus accumbens can be overcome by activation of TRPV1 receptors. Alcohol Clin Exp Res. 2014;38:2763–9.
Renteria R, Maier EY, Buske TR, Morrisett RA. Selective alterations of NMDAR function and plasticity in D1 and D2 medium spiny neurons in the nucleus accumbens shell following chronic intermittent ethanol exposure. Neuropharmacology. 2017;112:164–71.
Anderson ML, Nokia MS, Govindaraju KP, Shors TJ. Moderate drinking? Alcohol consumption significantly decreases neurogenesis in the adult hippocampus. Neuroscience. 2012;224:202–9.
Blanco AM, Guerri C. Ethanol intake enhances inflammatory mediators in brain: role of glial cells and TLR4/IL-1RI receptors. 2007:2616–30.
Pascual M, Baliño P, Alfonso-Loeches S, Aragón CMG, Guerri C. Impact of TLR4 on behavioral and cognitive dysfunctions associated with alcohol-induced neuroinflammatory damage. Brain Behav Immun. 2011;25:80–91.
Obernier JA, White AM, Swartzwelder HS, Crews FT. Cognitive deficits and CNS damage after a 4-day binge ethanol exposure in rats. Pharm Biochem Behav. 2002;72:521–32.
Pan B, Wang W, Zhong P, Blankman JL, Cravatt BF, Liu Q. Alterations of endocannabinoid signaling, synaptic plasticity, learning, and memory in monoacylglycerol lipase knock-out mice. J Neurosci. 2011;31:13420–30.
Ratano P, Petrella C, Forti F, Passeri PP, Morena M, Palmery M. et al. Pharmacological inhibition of 2-arachidonoilglycerol hydrolysis enhances memory consolidation in rats through CB2 receptor activation and mTOR signaling modulation. Neuropharmacology. 2018;138:210–8.
Lysenko LV, Kim J, Henry C, Tyrtyshnaia A, Kohnz RA, Madamba F, et al. Monoacylglycerol lipase inhibitor JZL184 improves behavior and neural properties in Ts65Dn mice, a model of Down syndrome. PLoS ONE 2014;9:e114521.
Rubio M, de Miguel R, Fernández-Ruiz J, Gutiérrez-López D, Carai MAM, Ramos JA. Effects of a short-term exposure to alcohol in rats on FAAH enzyme and CB1 receptor in different brain areas. Drug Alcohol Depend. 2009;99:354–8.
Steindel F, Lerner R, Häring M, Ruehle S, Marsicano G, Lutz B. et al. Neuron-type specific cannabinoid-mediated G protein signalling in mouse hippocampus. J Neurochem. 2013;124:795–807.
Basavarajappa BS, Hungund BL. Down-regulation of cannabinoid receptor agonist-stimulated [35S]GTPγS binding in synaptic plasma membrane from chronic ethanol exposed mouse. Brain Res. 1999;815:89–97.
Hamada N, Negishi Y, Mizuno M, Miya F, Hattori A, Okamoto N. et al. Role of a heterotrimeric G-protein, Gi2, in the corticogenesis: possible involvement in periventricular nodular heterotopia and intellectual disability. J Neurochem. 2017;140:82–95.
Basavarajappa BS, Saito M, Cooper TB, Hungund BL. Stimulation of cannabinoid receptor agonist 2-arachidonylglycerol by chronic ethanol and its modulation by specific neuromodulators in cerebellar granule neurons. Biochim Biophys Acta. 2000;1535:78–86.
García-Moreno LM, Cimadevilla JM. Acute and chronic ethanol intake: effects on spatial and non-spatial memory in rats. Alcohol 2012;46:757–62.
García-Moreno LM, Conejo NM, Capilla A, García-Sánchez O, Senderek K, Arias JL. Chronic ethanol intake and object recognition in young and adult rats. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:831–7.
Farr SA, Scherrer JF, Banks WA, Flood JF, Morley JE. Chronic ethanol consumption impairs learning and memory after cessation of ethanol. Alcohol Clin Exp Res. 2005;29:971–82.
Tanimizu T, Kono K, Kida S. Brain networks activated to form object recognition memory. Brain Res Bull. 2017;141:1–8.
Long JZ, Li W, Booker L, Burston JJ, Kinsey SG, Schlosburg JE. et al. Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Chem Rev. 2009;5:37–44.
Chen R, Zhang J, Wu Y, Wang D, Feng G, Tang YP, et al. Monoacilglycerol lipase is a new therapeutic target for Alzheimer’s disease. Cell Rep. 2012;27:320–31.
Acknowledgements
We thank all members of P. Grandes laboratory for their helpful comments, suggestions, and discussions during the performance of this study. The authors thank Giovanni Marsicano (INSERM, U1215 Neurocentre Magendie, Endocannabinoids and Neuroadaptation, Bordeaux, France. Université de Bordeaux, France), Beat Lutz (Institute of Physiological Chemistry and German Resilience Center, University Medical Center of the Johannes Gutenberg University Mainz, Germany), and Susana Mato (Achucarro Basque Center for Neuroscience, Science Park of the UPV/EHU, Leioa, Vizcaya, Spain) for providing the CB1 knockout mice.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Peñasco, S., Rico-Barrio, I., Puente, N. et al. Intermittent ethanol exposure during adolescence impairs cannabinoid type 1 receptor-dependent long-term depression and recognition memory in adult mice. Neuropsychopharmacol. 45, 309–318 (2020). https://doi.org/10.1038/s41386-019-0530-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-019-0530-5
This article is cited by
-
Consequences of adolescent drug use
Translational Psychiatry (2023)
-
Alcohol Consumption During Adolescence Alters the Cognitive Function in Adult Male Mice by Persistently Increasing Levels of DUSP6
Molecular Neurobiology (2023)
-
Design and validation of recombinant protein standards for quantitative Western blot analysis of cannabinoid CB1 receptor density in cell membranes: an alternative to radioligand binding methods
Microbial Cell Factories (2022)
-
Opioid System Contributes to the Trifluoromethyl-Substituted Diselenide Effectiveness in a Lifestyle-Induced Depression Mouse Model
Molecular Neurobiology (2021)
-
Fit-for-purpose based testing and validation of antibodies to amino- and carboxy-terminal domains of cannabinoid receptor 1
Histochemistry and Cell Biology (2021)