Abstract
Helminths have evolved sophisticated immune regulating mechanisms to prevent rejection by their mammalian host. Our understanding of how the human immune system responds to these parasites remains poor compared to mouse models of infection and this limits our ability to develop vaccines as well as harness their unique properties as therapeutic strategies against inflammatory disorders. Here, we review how recent studies on human challenge infections, self-infected individuals, travelers, and endemic populations have improved our understanding of human type 2 immunity and its effects on the microbiome. The heterogeneity of responses between individuals and the limited access to tissue samples beyond the peripheral blood are challenges that limit human studies on helminths, but also provide opportunities to transform our understanding of human immunology. Organoids and single-cell sequencing are exciting new tools for immunological analysis that may aid this pursuit. Learning about the genetic and immunological basis of resistance, tolerance, and pathogenesis to helminth infections may thus uncover mechanisms that can be utilized for therapeutic purposes.
Similar content being viewed by others
Introduction
Helminth infection models in mice have rapidly improved immunologists' understanding of type 2 immune responses, but an understanding of human immune responses to helminths and the ability to reduce the morbidity caused by helminth infections in endemic populations remains poor. Unique features of these parasites include being large multicellular organisms that mature through several larval stages, migrate through different tissues, and have been selected by evolution to produce immune evasion molecules. The type 2 response is particularly important for maintaining a balance between worm expulsion (i.e., resistance) as well as minimizing the virulence of these parasites (i.e., disease tolerance) by repairing the tissue damage caused by the worms.
A large proportion of helminth-infected individuals can be asymptomatic and morbidity typically affects individuals with high worm burdens, who may be more susceptible because of reduced immunity. Pathology also occurs in overly immune reactive individuals from collateral tissue damage, despite low worm burdens. This inter-individual natural variation in immune responses against helminths is poorly understood, but likely a result of interactions between genetic and environmental factors. If live helminths or helminth-produced molecules are to be tested as therapeutics for inflammatory diseases, understanding this heterogeneity in immune responses is likely necessary to maximize clinical benefit. Unfortunately, the most accessible readout of immune function in human populations is the peripheral blood, whereas the tissues in which the helminths reside are more difficult to access for analysis of tissue-resident immune cell phenotype and function.
Technological breakthroughs often precede major advances in the biological understanding of complex systems. Organoid technology to study epithelial cells from human biopsies, as well as the rapid advancement of single-cell sequencing technologies, are two promising tools with the potential to transform our knowledge of human mucosal responses. The ability to generate and maintain epithelial cells close to a native state from pinch biopsies collected from human subjects opens the door toward studying immune cell interactions with different types of epithelial cells. At the same time, single-cell sequencing provides transcriptional profiles by RNA-seq, surface molecules by CITE-seq, and epigenetic states by ATAC-sec, enabling not just phenotyping alone but an understanding of transcriptional regulation at a molecular level. In addition, the ability to freeze and thaw biopsy specimens collected during endoscopies, such that samples can be processed in batches rather than individually, to make organoids and for single-cell sequencing, has also been transformative. These exciting new approaches can now be deployed for clinical studies whereby it is possible to obtain intestinal biopsies, for example, in human challenge infections.
In this review, we discuss bridging the gap between mouse and human immunology of helminth infections, heterogeneity of responses in human populations, roles for the microbiome during helminth infections, and future opportunities in understanding human mucosal responses to helminths through technological advances and human challenge studies. While it is clear that immune responses elicited at tissue sites where pathogens are located are very different from what can be measured in the blood1, most studies of human helminth infections are based on the analysis of peripheral blood mononuclear cells (PBMCs). There are few studies on mucosal immune responses in humans during infection with gastro-intestinal helminth parasites. Consequently, tissue-resident cells such as ILC2, macrophages, and tissue-resident memory T cells are not well studied during human helminth infection. In contrast, mouse studies rarely characterize PBMCs and typically involve the analysis of samples from tissue sites and associated lymphoid tissue. Hence, there is quite a disconnect in our understanding of mouse and human responses to helminth infections.
There are logistical, technical, and ethical issues for accessing tissue samples to study helminth infection in resource low regions where these diseases are endemic. While we can technically obtain a biopsy from tissue sites, these approaches can be applied only when clinical infrastructure is sufficient and ethically justifiable. Experimental challenge helminth infection studies, as well as studies with self-infected individuals, provide the main glimpse of helminth responses in the gut. Perhaps in the future, studies in endemic regions can be coordinated between clinicians and scientists such that tissues harvested following intestinal or elective surgeries, may provide insights into immune responses elicited at tissue sites in helminth-infected individuals. In addition, endoscopies and imaging techniques might be tailored for immunology studies during helminth infection at tissue sites and associated lymphoid tissues2.
Mucosal responses to intestinal helminth infection in mice
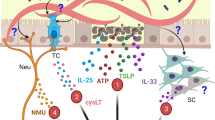
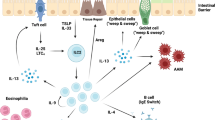
Based on gastro-intestinal helminth infection models in mice, the importance of type 2 immune response to expel and resist helminths is well appreciated. This type 2 immune response is characterized by the accumulation of cells like eosinophils, basophils, ILC2, mast cells, alternatively activated macrophages, and CD4+T helper 2 (TH2) cells, which produce effector type 2 cytokines (e.g., IL-4, IL-13) that changes the epithelial and associated stromal cells for clearance of the parasite. Signaling through IL-4R-alpha and STAT6 in intestinal epithelial cells is important for increasing epithelial cell turnover as part of the “weep and sweep” response, alongside stimulating increased mucus production by goblet cells and changes to the composition of this mucus barrier. This response maintains the mucosal barrier and prevents inflammatory responses triggered by gut bacteria. In addition, type 2 cytokines will increase the contraction of intestinal muscles, which together with the activation and release of mast cell proteases can increase the flow of fluids into the lumen to flush the helminths out of the intestinal tract3.
Over the last decade, the initiation of type 2 immune responses has become well defined4. Tuft cells, a chemosensory cell, were identified as being important in the production of activating cytokines like IL-255,6,7. Together with the release of IL-33 and TSLP, these alarmins are critical in the activation of type 2 immune response8,9,10,11,12. However, the role of these alarmins in human helminth infections is still understudied. Recently, “non-classical” type 2 cells like neutrophils have been shown to play important roles in parasite clearance and inflammation13,14,15,16. Neutrophils are particularly important during the early phases of helminth infection17, in the recruitment of other effector cells, as well as in directly killing the parasite, especially at the infective larva stage15. An important feature of this innate response is to promote tissue repair18. Notably, macrophages become alternatively activated by IL-4Ra/STAT6 signaling to adopt a phenotype that has enhanced anti-inflammatory tissue repair function. Type 2 cytokines will also increase immunoglobulin E production by B cells, which can activate basophils, eosinophils, and mast cells through Fc receptors to amplify type 2 cytokine production. Overall, this tissue repair response in the intestinal tract is important to maintain gut integrity and prevent the leakage of gut bacteria and sepsis.
While these are general features of type 2 responses to intestinal helminths, the different experimental models—Nippostronyglus brasilensis, Heligmosomoides polygyrus bakeri and Trichuris muris, have different lifecycles, reside in different parts of the intestine, hence function as models of different human helminth parasite with different pathogenicity and chronicity pattern19. The complex array of immune, epithelial, neuronal, and stromal changes has been previously reviewed4,19,20,21,22,23,24,25. Notably, there are also important immunometabolic consequences of helminth infections26,27. While in general the type 2 immune response is dominant during these infections, it is important to note that all infections also elicit to different degrees type 1 and type 17 cytokines that interact and cross-regulate the type 2 responses during acute and chronic infections.
Effects of helminth infections on human peripheral blood
Contrary to popular assumptions, human peripheral blood responses during natural helminth infection are usually characterized by a mixed population of type 2, regulatory, and type 1 immune cells28,29,30,31,32,33,34. Increased expression of type 2 cytokines, type 1 cytokines, regulatory cytokines, and markers such as CD161 and CTLA-4 is often observed in helminth-infected individuals compared to dewormed individuals28,29,32,35,36,37. Individuals with stronger type 2 cytokine responses are generally more resistant to re-infection and have lower worm burdens than those with a weaker type 2 response29,36,38,39, indicating a protective role for type 2 immune responses in parasite clearance and resistance to re-infection. Notably, the proportion of ILC2s is observed to be decreased in the PBMCs of helminth-infected individuals compared to the increase of CD4+ TH2cells28,40, which is in contrast to helminth-infected mice whereby ILC2s are increased following helminth infection in the tissues and lymphoid organs41,42,43,44,45. It is possible that ILC2s migrate from the peripheral blood to tissue sites during infection. In addition, helminth infection in endemic individuals is chronic; hence, the initial innate ILC2 response might have subsided and been replaced by the adaptive TH2 response, which is a different scenario from acute models of helminth infections in mice. Eosinophils by contrast are innate cells that increase in the blood, hence likely play a different role during helminth infection than ILC2s46,47,48. PBMCs of infected endemic individuals are also associated with increased T regulatory cell (Treg) function, including increased expression of immune checkpoint markers and production of regulatory cytokines31,32,49, which is especially prominent in children with high worm burden39. While these are general features for helminth infections, the different types of worms with different lifecycle and excretory-secretory products also result in a variety of responses that we shall consider below.
Helminth infections can be broadly separated into soil-transmitted intestinal helminths (including hookworm, the whipworm Trichuris trichiura, and roundworms such as Strongyloides stercoralis and Ascaris spp) from the tissue dwelling helminths that have intermediate hosts (e.g., insect vectors and snails) such as filarial parasites (e.g., Brugia malayi and Onchocerca volvulus) and flukes (e.g., Schistosoma spp). For hookworm infection, PBMCs of infected individuals are characterized by increased circulating Foxp3+ Treg cells that express markers such as CTLA-4 and GITR, as well as cytokines such as IL-10, transforming growth factor β (TGF-β), and IL-1731,32,35,49. Cellular and cytokine response are often mixed with higher type 2 cytokines like IL-13 and IL-5, inflammatory mediators like TNF-α and Interferon-γ as well as regulatory cytokines like IL-1034,36,49. S. stercoralis infection is also associated with higher circulating levels of type 2 cytokines like IL-4, IL-5, IL-9, and IL-13 and lower levels of type 1 cytokines like IFN-γ and TNF-α, which is reversed following deworming in infected individuals compared to uninfected individuals50. Infection with Ascaris lumbricoides is also skewed toward a type 2 response with increased production of type 2 cytokines in infected individuals51,52. In contrast, T. trichiura infection is characterized by a mixed immune response with skewing toward a regulatory and type 1 immune response52. More recently, we found that circulating levels of TGF-β were the strongest immune predictor of infection with T. trichiura53.
For tissue dwelling helminths such as filarial nematodes, an increased proportion of CD4+ CD25hi Treg cells in infected individuals is fairly typical33,54 and increased proportion of IL-13 producing ILC2 cells has been observed37. In vitro assays indicated that these Treg cells can suppress cytokine production and lymphocyte proliferative capacity, which may be important for protection33,55,56. For example, O. volvulus infection is characterized by T cell hypo-responsiveness to parasite antigen and an increase in regulatory response with the production of regulatory cytokines like IL-10 and TGF-β57. An increased proportion and expansion of adaptive Treg cells is also observed during infection with Schistosomes54,58,59. For these parasites, the Treg phenotype and proportion is correlated with the level of infection and age of the individual58,59. In vitro assays indicate that Treg cells can suppress parasite-specific responses including lymphocyte proliferative capacity and cytokine production60. During schistosomiasis, cytokine responses are also mixed and dependent on the stage and chronicity of the infection, with TH2 responses predominating during the chronic fibrotic stage of infection30 and persisting even after the infection is cleared61.
Hence, PBMC responses during infection with roundworms like Ascaris spp and Strongyloides spp are more skewed toward a type 2 response50,51, while infection with tissue dwelling helminths is more mixed with predominantly a Treg response, type 2 and type 1 immune response34,54. Cytokine profiles are also dependent on the age of the host and other factors including the chronicity of infection and co-infection with other pathogens29,34. Indeed, many factors contribute toward the heterogeneity of immune responses during helminth infections, including age of the individual during helminth infection29,39,58,59,62, host genetic factors63,64,65,66,67,68, presence of concurrent infection with another pathogen or parasite34,52,53,62, stage of the infection (whether acute or chronic phase)29,30,52,61, infectious dose and intensity29,39,69,70, presence of underlying inflammatory and/or autoimmune condition71,72, type of helminth parasite46,48 and/or the micro and macro-environment of the individual73. The relative contribution and interaction between these variables remain difficult to clearly establish. One approach that we have started to take to study the relative contributions of genetic and environmental variables toward heterogeneity in immune responses is to utilize “re-wilded” mice74,75, but that is a subject of a different review76.
Mucosal immune response during Trichuris trichiura infection
An individual self-infected with T. trichiura to treat his own symptoms of ulcerative colitis provided an opportunity to characterize intestinal immune responses during infection71. Consistent with previous reports77,78,79,80,81, biopsies from tissues with T. trichiura were characterized by infiltration of eosinophils and lymphocytes into the lamina propria and submucosa of the tissues by histopathologic analysis. The infiltration of lymphocytes might be associated with an increase in plasma cells in the tissue sites during T. trichiura infection, as overall T cell numbers can be unaltered in the intraepithelial tissue during T. trichiura infection78,82. However, the nature of the T cells in the tissues is altered during self-infection. Biopsies from a healthy subject self-infected with T. trichiura, showed an increase in type 2, regulatory, Th22, and even Th17 response in the cecum and colon72. Remission of symptoms in the individual with ulcerative colitis following a new infection with T. trichiura was also associated with Th2 cytokine production (IL-4) and Th22 T cell response (IL-22). Notably, a prominent Th17 T cell response associated with neutrophilic infiltration during symptomatic flares of ulcerative colitis was reduced by a new T. trichiura infection cycle. Type 2 cytokines and IL-22 may be important for goblet cell hyperplasia, increased mucous production the repair of epithelial cells and intestinal tissues71. Goblet cell hyperplasia has been reported in the cecum of those with T. trichiura infection77,81,82. In addition to eosinophils, increases in mast cells in the subepithelial region of children infected with T. trichiura from rectal biopsy of lesions from parasitized individuals indicate that the release of histamine plays a role during T. trichiura infection in humans83. TNF-α is another cytokine that could synergize with type 2 cytokines to mediate resistance to Trichuris parasites84. Immunohistochemistry staining of the lamina propria of children with whipworm infection shows an increase in TNF-α positive cells. There is also increased secretion of TNF-α in cultures of colonic biopsies, as well as more TNF-α in plasma levels of infected children85. Foxp3+ T cell abundance is increased in inflamed tissues during T. trichiura infection72, and in regions inflamed from colitis symptoms, but is not associated with infection above what is already observed in the context of ulcerative colitis71. Increased abundance of Foxp3+ T cells is often associated with any type of inflammation in the intestines but the regulation and function of different types of intestinal regulatory T cells is complex86,87. It should be emphasized that such studies on self-infected individuals are limited by the uncertain dosage and source of parasite material for these uncontrolled observations, as well as ethical considerations. Hence, the alternative of experimental human helminth infection studies is considered below.
Immune responses during experimental helminth infection challenge
Human helminth challenge infections with Necator americanus88 and S. mansoni89 are being conducted in countries where helminth infections are no longer endemic as a platform for testing vaccines and drugs, but also to understand the pathogenesis and immune responses to helminth infections. In addition, the pig whipworm T. suis has also been tested in human subjects as a therapeutic for inflammatory bowel disease and other autoimmune diseases90. For safety reasons, either low dosage of larvae for N. americanus, or male-only S. mansoni cercariae that do not produce pathogenic eggs89 are utilized. Study volunteers are then cured with anthelminthic drug treatment after the studies, although some choose not to be treated. Since T. suis does not establish patent infection in humans, no treatment is necessary for studies with Trichuris suis ova (TSO). Many of these studies have been done in the context of autoimmune disorders71,91,92,93 and many of these challenge infection studies have been recently reviewed94,95,96. As expected, a general feature is increased type 2 cytokines such as IL-4, IL-5, and IL-13 being made from cultured PBMCs from infected individuals compared to uninfected individuals97,98 as well as increased eosinophilia in the peripheral blood72,98,99.
There have been the most controlled human infection studies with the hookworm N. americanus, which provides opportunities to characterize biopsy specimens from the duodenum in a few examples. As expected, challenge infection is associated with eosinophilia98,99,100, production of type 2 cytokines97,98,100, but also other pro-inflammatory and regulatory cytokines like IL-2, IL-10, IFN-γ as well as IL-17A following stimulation of PBMCs isolated infected volunteers97. Consistent with these peripheral responses, mucosal responses at the infected tissue site in the gut mucosa has a mixed response made up of a strong type 2, regulatory and some type 1 responses97. An increase in mucosal cytokines of IL-23 and IL-22 was also observed97 and intense eosinophilic infiltration into the mucosa similar to natural infections100,101. Controlled infection studies were performed in the context of Celiac Disease, whereby infection was associated with a reduction in IFN gamma and IL-17 producing T cells and cytokines and the expansion of CD4+ FoxP3+ T cells expressing CTLA-4 with the production of IL-10 in the mucosal tissue sites91,102. This was also associated with duodenal eosinophilia100.
For S. mansoni, controlled human infection is still at an early stage, with a study among naive volunteers in the Netherlands89; hence, the immunological analysis has only been done in this study. Despite the lack of pathogenic egg production because of infection with male cercariae only103, some study subjects still experienced an acute inflammatory response known as Katayama syndrome104, especially with a higher dose of parasites89. Nonetheless, there is interest in implementing this strategy to accelerate vaccine development in endemic countries such as Uganda105. As expected, there is seroconversion for IgM and IgG over time after infection and an interesting trend toward higher IgG1 levels in subjects with Katayama syndrome89. Indeed, volunteers that develop this acute symptom were associated with higher immune responses overall, including both Th2 cytokines, but also Th1 cytokines such as IFNg, IP-10, and MIP-1B. It is still unclear why some volunteers develop acute symptoms while others do not89.
Challenge infection with pig whipworm eggs, TSO, which is well tolerated in humans with some success in treatment of inflammatory bowel disease has shown variable immunological outcomes in patients106,107,108,109,110,111. Sometimes, modulation of the Th1/Th2 balance and innate response is seen107 and sometimes not106. In the most recent report, during infection with TSO, changes in the B cell responses and activation of the T cell pool were seen; however, no significant changes in the proportion of other innate cell population, and the balance between Th1/Th2 cell were noticeable following immunophenotyping of PBMCs by mass spectrometry106.
Although epithelial cell changes such as goblet cell hyperplasia clearly occur during challenge infection, with the recent knowledge of epithelial cell responses critical in murine model of helminth infection112, it will be interesting to determine whether tuft cell responses in humans exhibit similar biology. In addition to the small number of study subjects investigated, the systemic and mucosal immune response during challenge helminth infection is also complicated by heterogeneity of the underlying disease conditions that are being studied. Nonetheless, detailing the host response in great depth for a small number of subjects can still provide substantial insights into unique features of helminth infection on the human intestinal immune response.
New approaches for studying human mucosal responses to helminths
There are currently multiple technological improvements that have the potential to be transformative for our understanding of human mucosal responses. Here we highlight several that could be applied to helminth infections. Analysis of mucosal biopsies has been difficult partly because it requires the processing of fresh tissues that is time consuming and dependent on the time of clinical procedures. Hence, the development of approaches to immediately cryopreserve mucosal biopsy tissues113,114 greatly expands the capacity for detailed immunophenotyping of tissue-resident cells, as well as the generation of intestinal organoids from individual patients for mechanistic experiments115. Being able to study epithelial cells in organoid systems close to a native state should enable investigating immune cell interactions with these cells in co-culture systems.
While single-cell sequencing technologies have yet to be fully applied to helminth infections in human subjects, it has already enabled the identification of many new cell populations in the intestinal mucosa during inflammatory bowel diseases and changed our understanding of complexity and heterogeneity of this disease116. As this technology continues to improve, sequencing does not just provide transcriptional profiles by RNA-seq, but can validate protein expression of molecules expressed on the cell surface by CITE-seq, as well as epigenetic states of the cells by ATAC-seq. Characterization of epigenetic state and transcription in the same cells will further enable dissection of transcriptional regulation at a molecular level. In addition, V(D) J sequencing enables the assessment of clonal expansion and BCR and TCR usage; hence, allowing us to gain unprecedented insights into immune response of different organ systems.
The intestinal immune system has been well characterized at a single cell level117. This includes samples from in utero intestinal developmental tissues118, but has primarily centered on steady-state conditions and inflammatory bowel diseases117. There have been studies examining the response to the gut microbiota119; however, intestinal immune responses against eukaryotic pathogens remain to be explored by single-cell sequencing. Hence we have a good view of the cellular landscape of the human intestinal immune system at steady state and during IBD, but not for other types of intestinal infections including helminths. Nonetheless, the detailed atlas of immune and non-immune cells in the intestine already available at steady state, including the interactions with the neuronal system120, will facilitate mapping any new data generated during human challenge infection studies onto a well-established road-map to identify novel signatures that are helminth specific. In mouse models of helminth infection, intestinal CD4+ T cells121 and epithelial cells122 have been examined, indicating interesting signatures of goblet cell, tuft cell, and TH2 cell expansion and phenotypes, which would be important to confirm in a human setting.
Organoid technology is already improving our understanding of epithelial cell responses to helminth infections in mouse models123, including the ability of helminths to regulate or inhibit appropriate epithelial cell responses124, in combination with scRNA-seq122. The revolution in understanding of tuft cell biology driven by helminth infections has been driven by mouse intestinal organoids5,6,7,125, but this has not yet transferred over to the human setting. Recently, a role for macrophage migration inhibitory factor in expanding intestinal tuft cells126 has also been investigated using a combination of in vivo mouse models and organoid systems. As another example, caecaloids have been developed in mice127 that can be used to examine early infection events by the mouse whipworm T. muris, which could potentially be replicated in the human system to study T. trichiura in an in vitro setting.
However, organoid structures for other tissues and organs have yet to be deeply exploited to study host interactions with helminth infections. Tumor organoids for example128, have become sophisticated models of the tumor microenvironment, which can be combined with fibroblasts and immune cells to examine these complex interactions. There are many similarities between the immunoregulatory features of certain tumor microenvironments and the consequences of helminth infections129, especially in the tissues. In combination with CRISPR-based gene modification130, the functions of specific molecules in shaping the host-helminth interaction not just in the intestinal epithelium but in other organ systems can be examined genetically. In the rapidly expanding field of immune-oncology, tumor organoids are being used as models to optimize T cell responses against cancers, when combined with other cells from the tumor microenvironment131. There are exciting opportunities to apply such experimental strategies to study human responses to helminth infections, as such in vitro systems can now be generated from human biopsy materials and immune cells.
From the perspective of personalized medicine, biopsies obtained to produce patient-specific organoids for analysis could potentially predict helminth mediated effects on inflammatory bowel disease patients, which have very heterogeneous responses to different treatment options. While such studies are still hypothetical, it is clear that the combination of reductionist in vitro systems such as the organoids, coupled with complex single-cell sequencing analysis of ex vivo patient samples, will have the potential to help us understand heterogeneity in human intestinal immune responses to helminth infections.
Effects of helminths on microbiome during natural infection
The microbiota plays a critical role in regulating the mucosal immune system and during intestinal helminth infection, these worms must interact with the gut bacteria and their metabolites. These complex interactions likely determine the establishment of helminth colonization, expulsion of the parasites, disease severity, and host immune-modulation. As we discuss the heterogeneity of human responses to helminth infection, it is important to discuss the role that interactions between the gut microbiota and helminths may play in this process. Understanding these interactions may provide new strategies for alleviating human morbidity resulting from helminth infections in endemic regions and emerging challenges such as anthelminthic drug resistance132.
Most of the work in this area involves describing associations between intestinal helminths and fecal samples by 16S ribosomal sequencing analysis in endemic populations. These include cross-sectional (n = 12)53,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147 and longitudinal studies (n = 10)148,149,150,151,152,153,154,155,156,157,158,159, but the conclusions are often divergent, due to differences in the prevalence of helminths and study population for each analysis (see below). A common feature observed from some cross-sectional studies is increased gut microbial diversity in helminth-infected individuals133,138,142,144,145,146,151,153,159; however, some other studies showed either reduced diversity146 or no significant differences in microbial diversity in infected individuals134,135,139,140,143,148,154. A recent effort to conduct a meta-analysis suggested helminths that colonized the large intestine, i.e., T. trichiura and Enterobius vermicularis are more likely to increase microbial diversity and alter microbial composition160. While the bacteria associated with helminth infections varies between studies, some commonly reported organisms fall in the order Clostridiales136,139,155, order Bacteroidales136,138,155, family Paraprevotellaceae144,151, family Lachnospiracaea140,153, and Bacteroides enterotype141,142.
As the gut microbiota varies across geography, ethnicity and mode of subsistence161, in studies conducted in various continents including South America53,141,157, North America136,147, Africa133,138,139,148,153,154,156, Europe151, and Asia134,135,140,142,143,144,145,146,149,150,152,153,155,158,159, it is not surprising to have divergent findings. In addition, studies have focused on different parasites, based on the study population including T. trichiura133,144,149,155,157, A. lumbricoides147, S. haematobium139,156, S. mansoni154 S. japonicum134, Strongyloides stercoralis151, Clonorchis sinensis142, Haplorchis taichui146, E. vermicularis159, or mixed infections136,138,140,141,143,145,148,150,152,153,158. Differences in lifecycle and physical location in the intestine would likely affect the gut bacterial interactions160. Analyzing the composition of mucosal bacteria may be more informative for helminths (e.g., A. lumbricoides and hookworm) living in the small intestine.
Longitudinal studies to examine the effect of deworming on gut microbiota may provide more insight into cause and effect relationships, but are also potentially confounded by direct impact of anthelmintic treatment on the gut microbiota. While some studies observed differences in gut microbial diversity following deworming treatment148,151,155, others observed no impact from treatment152,154,157. The microbiota may also affect treatment efficacy, as the treatment outcome of the combination of albendazole and ivermectin was associated with pre-treatment enterotype, with increased egg reduction in Enterotype 3, which is enriched with Ruminococcus torques & Eubacterium coprostanoligenes158.
There may also be technical factors explaining the variability seen in these studies. For example, the bioinformatic approach used for the microbiota analysis can introduce technical limitations. Most studies utilize 16S rRNA sequencing133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158, while a few have utilized shotgun metagenomics138,140,153,158. 16S sequencing only provides a portion of the gut microbiota profile162 and does not provide genome data to infer microbial functional, which can be generated by metagenomics. However, shotgun metagenomic analyses are limited by available reference databases, which often do not include data from underrepresented groups that are helminth infected. Metagenomic data can provide insights into other eukaryotes, which is understudied. Such transkingdom interactions were investigated by Partida-Rodriguez et al. in a parasite-infected mother-child cohort study in a semirural community in Mexico136. Although intestinal helminth infection was not associated with other eukaryotes, there was a positive correlation between bacterial and other eukaryotic taxa (e.g., fungus Candida with Bacteroides and Actinomyces, Bifidobacterium and Prevotella copri)136. Questions surrounding interactions between helminths with viruses (virome), fungi (mycobiome), and archaea (archaeome) remain to be characterized and would be of potential interest in the field in future studies.
Despite the importance of helminths and the microbiota in regulating immune responses, there are just a few human studies that have examined this three-way relationship53,138,149,150,159. In Indonesia, Martin et al. observed that higher microbial diversity was associated with greater IFN-γ responses to PHA in helminth-negative individuals when compared to helminth-positive individuals150. There was also a negative association between proportions of Bacteroidetes and IL-10 responses to LPS in uninfected individuals and helminth infection diminished this effect150. Among an indigenous population in Malaysia, we found blood transcriptional profiles that were associated with T. trichiura infection149. In this study, serum zinc and iron levels were affected by helminth infection status, independent of dietary metal intake, and these serum zinc and iron levels were also associated with an abundance of specific microbial taxa149. A study in Cameroon also identified associations between cytokine responses, intestinal helminths, and the gut microbiota138, whereas a study in Columbia identified circulating TGF-β as the strongest predictor of the T. trichiura egg burden in a cross-sectional study53. There has also been one study examining secreted IgA levels in E. vermicularis infected schoolchildren in Taiwan159.
Overall, our understanding of the three-way interaction between helminths, the microbiota, and immune responses remains poor and most studies are restricted to the analyses of peripheral blood and stool samples from study participants. Metabolites are an important regulator of the crosstalk between the immune system and the microbiome163,164, and while many mouse studies have uncovered a role for microbial metabolites in regulating responses to helminthes27,this area remains understudied in human infection. For example, helminth infection is associated with increased short-chain fatty acid production and Clostridiales organisms that have important immunoregulatory properties165,166, and additional studies are needed to firmly establish this relationship in human populations.
Effects of helminths on microbiome during challenge infection
Controlled experimental challenge infections may be an approach to minimize confounding factors and determine more direct effects of helminths on the microbiome and mucosal responses. The hookworm, N. americanus infection studies have been conducted on healthy individuals167,168, participants with relapsing multiple sclerosis169, and coeliac disease170. A study on 8 healthy volunteers infected with 20 L3 larvae found no major impact on the gut microbiota at 8 weeks post infection167. A more recent study on 20 healthy young volunteers with higher dosages (i.e., 50, 100, or 150) of L3 found that the bacterial richness increased significantly during the established infection phase (week 8–20), but not during the acute infection phase (trial week 0–8)168. As hookworm resides in the small intestine, these results are consistent with deworming studies indicating that these worms may not alter the gut microbiota significantly, especially in the first 8 weeks after the infection.
One of the goals for developing challenge infection models is to determine if helminth infection can have therapeutic benefits for autoimmune diseases171. A study on 24 relapsing multiple sclerosis patients infected with 25 L3 larvae and 26 patients on placebo treatment observed greater gut microbial diversity in infected individuals compared with the placebo group169. There were also significant differences in some bacterial taxa with putative immune-modulatory between groups169. In a rare study with access to biopsy material, 12 patients with coeliac disease were challenged with 20 L3 larvae and the microbiota of the duodenum tissue was analyzed170. There was greater microbial richness and diversity of tissue-adherent microbiota after exposure to N. americanus. Overall, infection with N. americanus may perhaps restore some microbial diversity to patients with inflammatory conditions, even if it does not have a large effect on the microbiota of healthy study participants.
Summary
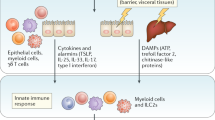
Helminths and the microbiota have co-evolved with their mammalian hosts to exist primarily under homeostatic asymptomatic conditions. Pathogenesis and disease morbidity arises when this homeostasis fails, which could be a result of heterogeneity in immune responses directed against the worms, composition of the microbial communities present, or genetic diversity in the worm population. We hypothesize that under steady-state conditions, the regulatory and the type 2 response, as well as some type 17 cytokines such as IL-22, helps maintain the mucus barrier to prevent bacterial translocation and increased inflammation. Conditions that result in either too strong or too weak a response against the parasites will result in aberrant inflammatory responses that are damaging to the host, either from an overwhelming parasite burden or collateral tissue damage. A better understanding of immune mechanisms that regulate this balance in humans is limited by our ability to access tissue samples, as peripheral blood is more easily characterized. However, recent advances in technology and clinical studies with challenge infections should begin to fill in many of the gaps in understanding the differences between mechanisms at play in the human population, compared to what has been learnt from mouse models of infection. In addition, understanding how heterogeneity in human immune responses to helminth infections could be modulated by the gut microbiota will also be critical (Fig. 1).
A systems immunology approach toward understanding inter-individual immune variation to helminth infection can uncover new mechanisms for therapy and vaccination. Clinical research (a) on helminth infected subjects from well-characterized study cohorts with careful documentation of environmental metadata provides the basis for infrastructure required for appropriate sample acquisition (b). The development of successful freezing protocols has enabled biobanking blood and fecal samples, as well as more recently, tissue biopsy material, which can now by analyzed at a later stage (c) under state-of-the-art laboratory conditions. Spectral flow cytometry (>40 parameters), as well as single-cell-sequencing analysis, of blood and tissue samples enables detailed phenotyping of cellular composition and function but can also enable understanding of transcriptional regulation at a molecular level in combination with whole genome sequencing. Fecal samples can undergo metagenomic and metatranscriptomic sequencing analyses to determine microbial composition and function, as well as metabolomic analyses to identify metabolites that may influence the immune system of the subjects. d Data analyses by bioinformatics and computational biology approaches utilizing new algorithms for calculating effect sizes and visualizing large data sets can enable insights into drivers of inter-individual immune variation (e). Immune responses to helminth infections typically fall within a normal distribution curve (f) with individuals who fall at both tails of the distribution curve having the potential for pathology resulting from super infection or immune pathology. Understanding this immune distribution may enable better selection of subjects for clinical trials or challenge infection studies (g) that will minimize unexpected adverse events and maximize potential therapeutic benefits. Created with BioRender.com.
References
Farber, D. L. Tissues, not blood, are where immune cells function. Nature 593, 506–509 (2021).
O’Sullivan, J. D. B. et al. X-ray micro-computed tomography (μCT): an emerging opportunity in parasite imaging. Parasitology 145, 848–854 (2018).
Artis, D. & Grencis, R. K. The intestinal epithelium: sensors to effectors in nematode infection. Mucosal Immunol. 1, 252–264 (2008).
Sorobetea, D., Svensson-Frej, M. & Grencis, R. Immunity to gastrointestinal nematode infections. Mucosal Immunol. 11, 304–315 (2018).
Gerbe, F. et al. Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature 529, 226–230 (2016).
Howitt, M. R. et al. Tuft cells, taste-chemosensory cells, orchestrate parasite type 2 immunity in the gut. Science 351, 1329–1333 (2016).
von Moltke, J., Ji, M., Liang, H. E. & Locksley, R. M. Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature 529, 221–225 (2016).
Massacand, J. C. et al. Helminth products bypass the need for TSLP in Th2 immune responses by directly modulating dendritic cell function. Proc. Natl Acad. Sci. USA 106, 13968–13973 (2009).
Taylor, B. C. et al. TSLP regulates intestinal immunity and inflammation in mouse models of helminth infection and colitis. J. Exp. Med. 206, 655–667 (2009).
Humphreys, N. E., Xu, D., Hepworth, M. R., Liew, F. Y. & Grencis, R. K. IL-33, a potent inducer of adaptive immunity to intestinal nematodes. J. Immunol. 180, 2443–2449 (2008).
Wills-Karp, M. et al. Trefoil factor 2 rapidly induces interleukin 33 to promote type 2 immunity during allergic asthma and hookworm infection. J. Exp. Med. 209, 607–622 (2012).
Hung, L.-Y. et al. IL-33 drives biphasic IL-13 production for noncanonical Type 2 immunity against hookworms. Proc. Natl Acad. Sci. USA 110, 282–287 (2013).
Chen, F. et al. Neutrophils prime a long-lived effector macrophage phenotype that mediates accelerated helminth expulsion. Nat. Immunol. 15, 938–946 (2014).
Sutherland, T. E. et al. Chitinase-like proteins promote IL-17-mediated neutrophilia in a tradeoff between nematode killing and host damage. Nat. Immunol. 15, 1116–1125 (2014).
Bouchery, T. et al. Hookworms evade host immunity by secreting a deoxyribonuclease to degrade neutrophil extracellular traps. Cell Host Microbe 27, 277–289.e6 (2020).
Pesce, J. T. et al. Neutrophils clear bacteria associated with parasitic nematodes augmenting the development of an effective Th2-type response. J. Immunol. 180, 464–474 (2008).
Ajendra, J. Lessons in type 2 immunity: neutrophils in helminth infections. Semin. Immunol. 53, 101531 (2021).
Allen, J. E. & Wynn, T. A. Evolution of Th2 immunity: a rapid repair response to tissue destructive pathogens. PLoS Pathog. 7, e1002003 (2011).
Zaph, C., Cooper, P. J. & Harris, N. L. Mucosal immune responses following intestinal nematode infection. Parasite Immunol. 36, 439–452 (2014).
Allen, J. E. & Maizels, R. M. Diversity and dialogue in immunity to helminths. Nat. Rev. Immunol. 11, 375–388 (2011).
Gazzinelli-Guimaraes, P. H. & Nutman, T. B. Helminth parasites and immune regulation. F1000Research 7, F1000 Faculty Rev-685 (2018).
Harris, N. L. & Loke, P. Recent advances in type-2-cell-mediated. Immunity 47, 1024–1036 (2017).
Maizels, R. M. & McSorley, H. J. Regulation of the host immune system by helminth parasites. J. Allergy Clin. Immunol. 138, 666–675 (2016).
Grencis, R. K., Humphreys, N. E. & Bancroft, A. J. Immunity to gastrointestinal nematodes: mechanisms and myths. Immunological Rev. 260, 183–205 (2014).
Mair, I., Else, K. J. & Forman, R. Trichuris muris as a tool for holistic discovery research: from translational research to environmental bio-tagging. Parasitology 148, 1722–1734 (2021).
van der Zande, H. J. P., Zawistowska-Deniziak, A. & Guigas, B. Immune regulation of metabolic homeostasis by helminths and their molecules. Trends Parasitol. 35, 795–808 (2019).
Moyat, M., Coakley, G. & Harris, N. L. The interplay of type 2 immunity, helminth infection and the microbiota in regulating metabolism. Clin. Transl. Immunol. 8, e01089 (2019).
de Ruiter, K. et al. Helminth infections drive heterogeneity in human type 2 and regulatory cells. Sci. Transl. Med. 12, eaaw3703 (2020).
Faulkner, H. et al. Age- and infection intensity-dependent cytokine and antibody production in human trichuriasis: the importance of IgE. J. Infect. Dis. 185, 665–672 (2002).
Caldas, I. R. et al. Human schistosomiasis mansoni: immune responses during acute and chronic phases of the infection. Acta Tropica 108, 109–117 (2008).
Geiger, S. M., Massara, C. L., Bethony, J., Soboslay, P. T. & Corrêa-Oliveira, R. Cellular responses and cytokine production in post-treatment hookworm patients from an endemic area in Brazil. Clin. Exp. Immunol. 136, 334–340 (2004).
Ricci, N. D. et al. Induction of CD4+CD25+FOXP3+ regulatory T cells during human hookworm infection modulates antigen-mediated lymphocyte proliferation. PLoS Negl. Trop. Dis. 5, e1383 (2011).
Wammes, L. J. et al. Regulatory T cells in human lymphatic filariasis: stronger functional activity in microfilaremics. PLoS Negl. Trop. Dis. 6, e1655 (2012).
Pit, D., Polderman, A., Baeta, S., Schulz-Key, H. & Soboslay, P. Parasite-specific antibody and cellular immune responses in humans infected with Necator americanus and Oesophagostomum bifurcum. Parasitol. Res. 87, 722–729 (2001).
Wammes, L. J. et al. Community deworming alleviates geohelminth-induced immune hyporesponsiveness. Proc. Natl Acad. Sci. USA 113, 12526–12531 (2016).
Quinnell, R. J., Pritchard, D. I., Raiko, A., Brown, A. P. & Shaw, M.-A. Immune responses in human necatoriasis: association between interleukin-5 responses and resistance to reinfection. J. Infect. Dis. 190, 430–438 (2004).
Boyd, A., Ribeiro, J. M. C. & Nutman, T. B. Human CD117 (cKit)+ innate lymphoid cells have a discrete transcriptional profile at homeostasis and are expanded during filarial infection. PLoS ONE 9, e108649 (2014).
Jackson, J. A. et al. T helper cell type 2 responsiveness predicts future susceptibility to gastrointestinal nematodes in humans. J. Infect. Dis. 190, 1804–1811 (2004).
Turner, J. D. et al. Th2 cytokines are associated with reduced worm burdens in a human intestinal helminth infection. J. Infect. Dis. 188, 1768–1775 (2003).
Nausch, N. et al. Group 2 innate lymphoid cell proportions are diminished in young helminth infected children and restored by curative anti-helminthic treatment. PLoS Negl. Trop. Dis. 9, e0003627 (2015).
Fallon, P. G. et al. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J. Exp. Med 203, 1105–1116 (2006).
Neill, D. R. et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature 464, 1367–1370 (2010).
Price, A. E. et al. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc. Natl Acad. Sci. USA 107, 11489–11494 (2010).
Moro, K. et al. Innate production of TH2 cytokines by adipose tissue-associated c-Kit+Sca-1+ lymphoid cells. Nature 463, 540–544 (2010).
Herbert, D. B. R., Douglas, B. & Zullo, K. Group 2 innate lymphoid cells (ILC2): type 2 immunity and helminth immunity. Int. J. Mol. Sci. 20, 2276 (2019).
Gabrie, J. A., Rueda, M. M., Rodríguez, C. A., Canales, M. & Sanchez, A. L. Immune profile of honduran schoolchildren with intestinal parasites: the skewed response against geohelminths. J. Parasitol. Res. 2016, 1769585 (2016).
Huang, L. & Appleton, J. A. Eosinophils in helminth infection: defenders and dupes. Trends Parasitol. 32, 798–807 (2016).
Wright, V. J. et al. Early exposure of infants to GI nematodes induces Th2 dominant immune responses which are unaffected by periodic anthelminthic treatment. PLoS Negl. Trop. Dis. 3, e433 (2009).
Phasuk, N., Apiwattanakul, N., & Punsawad, C. Profiles of CD4+, CD8+, and regulatory T cells and circulating cytokines in hookworm-infected children in southern Thailand. Med. Microbiol. Immunol. 211, 19–28 (2021).
Anuradha, R. et al. Systemic cytokine profiles in strongyloides stercoralis infection and alterations following treatment. Infect. Immun. 84, 425–431 (2015).
Cooper, P. J. et al. Human infection with ascaris lumbricoides is associated with a polarized cytokine response. J. Infect. Dis. 182, 1207–1213 (2000).
Figueiredo, C. A. et al. Chronic intestinal helminth infections are associated with immune hyporesponsiveness and induction of a regulatory network. Infect. Immun. 78, 3160–3167 (2010).
Easton, A. V. et al. Immune response and microbiota profiles during coinfection with Plasmodium vivax and soil-transmitted helminths. mBio 11, e01705–20 (2020).
Metenou, S. et al. At homeostasis filarial infections have expanded adaptive T regulatory but not classical Th2 cells. J. Immunol. 184, 5375–5382 (2010).
Babu, S. et al. Human type 1 and 17 responses in latent tuberculosis are modulated by coincident filarial infection through cytotoxic T lymphocyte antigen–4 and programmed death–1. J. Infect. Dis. 200, 288–298 (2009).
King, C. L. et al. Cytokine control of parasite-specific anergy in human lymphatic filariasis. Preferential induction of a regulatory T helper type 2 lymphocyte subset. J. Clin. Investig. 92, 1667–1673 (1993).
Doetze, A. et al. Antigen-specific cellular hyporesponsiveness in a chronic human helminth infection is mediated by T(h)3/T(r)1-type cytokines IL-10 and transforming growth factor-beta but not by a T(h)1 to T(h)2 shift. Int Immunol. 12, 623–630 (2000).
Nausch, N., Midzi, N., Mduluza, T., Maizels, R. M. & Mutapi, F. Regulatory and activated T cells in human schistosoma haematobium infections. PLoS ONE 6, e16860 (2011).
Milner, T. et al. Circulating cytokine levels and antibody responses to human Schistosoma haematobium: IL-5 and IL-10 levels depend upon age and infection status. Parasite Immunol. 32, 710–721 (2010).
Grogan, J. L., Kremsner, P. G., Deelder, A. M. & Yazdanbakhsh, M. Antigen-specific proliferation and interferon-gamma and interleukin-5 production are down-regulated during Schistosoma haematobium infection. J. Infect. Dis. 177, 1433–1437 (1998).
Soonawala, D., Geerts, J.-W. H. J., de Mos, M., Yazdanbakhsh, M. & Visser, L. G. The immune response to schistosome antigens in formerly infected travelers. Am. J. Trop. Med. Hyg. 84, 43–47 (2011).
Sanchez, A. L., Mahoney, D. L. & Gabrie, J. A. Interleukin-10 and soil-transmitted helminth infections in Honduran children. BMC Res Notes 8, 55 (2015).
Williams‐Blangero, S. et al. Genetic component to susceptibility to Trichuris trichiura: evidence from two Asian populations. Genet. Epidemiol. 22, 254–264 (2002).
Williams-Blangero, S. et al. Two quantitative trait loci influence whipworm (Trichuris trichiura) infection in a Nepalese population. J. Infect. Dis. 197, 1198–1203 (2008).
Williams-Blangero, S. et al. Localization of multiple quantitative trait loci influencing susceptibility to infection with Ascaris lumbricoides. J. Infect. Dis. 197, 66–71 (2008).
Williams-Blangero, S. et al. Genetic analysis of susceptibility to infection with Ascaris lumbricoides. Am. J. Trop. Med. Hyg. 60, 921–926 (1999).
Williams-Blangero, S. et al. Genes on chromosomes 1 and 13 have significant effects on Ascaris infection. Proc. Natl Acad. Sci. USA 99, 5533–5538 (2002).
Costa, R. D. S. et al. Effect of polymorphisms on TGFB1 on allergic asthma and helminth infection in an African admixed population. Ann. Allergy, Asthma Immunol. 118, 483–8.e1 (2017).
Colombo, S. A. P. & Grencis, R. K. Immunity to soil-transmitted helminths: evidence from the field and laboratory models. Fron. Immunol. 11, 1286 (2020).
Turner, J. D. et al. Intensity of intestinal infection with multiple worm species is related to regulatory cytokine output and immune hyporesponsiveness. J. Infect. Dis. 197, 1204–1212 (2008).
Broadhurst, M. J. et al. IL-22+ CD4+ T cells are associated with therapeutic trichuris trichiura infection in an ulcerative colitis patient. Sci. Transl. Med. 2, 60ra88–60ra88 (2010).
Dige, A. et al. Mucosal and systemic immune modulation by Trichuris trichiura in a self-infected individual. Parasite Immunol. 39, e12394 (2017).
Leung, J. M. et al. Rapid environmental effects on gut nematode susceptibility in rewilded mice. PLoS Biol. 16, e2004108 (2018).
Gause, W. C., Rothlin, C. & Loke, P. Heterogeneity in the initiation, development and function of type 2 immunity. Nat. Rev. Immunol. 20, 603–614 (2020).
Lin, J.-D. et al. Rewilding Nod2 and Atg16l1 mutant mice uncovers genetic and environmental contributions to microbial responses and immune cell composition. Cell Host Microbe 27, 830–840.e4 (2020).
Oyesola, O. O., Souza, C. O. S. & Loke. P. The influence of genetic and environmental factors and their interactions on immune response to helminth infections. Front. Immunol. 13, 869163 (2022).
Bundy, D. A. P. & Cooper, E. S. Trichuris and Trichuriasis in humans. In Advances in Parasitology (eds Baker, J. R. & Muller, R.) 107–173 (Academic Press, 1989).
Khuroo, M. S., Khuroo, M. S. & Khuroo, N. S. Trichuris dysentery syndrome: a common cause of chronic iron deficiency anemia in adults in an endemic area (with videos). Gastrointest. Endosc. 71, 200–204 (2010).
Kaminsky, R. G., Castillo, R. V. & Flores, C. A. Growth retardation and severe anemia in children with Trichuris dysenteric syndrome. Asian Pac. J. Trop. Biomed. 5, 591–597 (2015).
Arean, V. & Crandall, C. Ascariasis. Pathology of Protozoal and Helminthic Diseases. (ed. Marcial-Rojas, R. A.) 769–807 (Williams & Wilkins Co., Baltimore, 1971).
Kaur, G., Raj, S. M. & Naing, N. N. Trichuriasis: localized inflammatory responses in the colon. Southeast Asian J. Trop. Med Public Health 33, 224–228 (2002).
MacDonald, T. T. et al. Histopathology and immunohistochemistry of the caecum in children with the Trichuris dysentery syndrome. J. Clin. Pathol. 44, 194–199 (1991).
Cooper, E. S. et al. Immediate hypersensitivity in colon of children with chronic Trichuris trichiura dysentery. Lancet 338, 1104–1107 (1991).
Artis, D. et al. Tumor necrosis factor alpha is a critical component of interleukin 13-mediated protective T helper cell type 2 responses during helminth infection. J. Exp. Med 190, 953–962 (1999).
MacDonald, T. T. et al. 3. Mucosal macrophages and cytokine production in the colon of children with Trichuris trichiura dysentery. Trans. R. Soc. Trop. Med. Hyg. 88, 265–268 (1994).
Tanoue, T., Atarashi, K. & Honda, K. Development and maintenance of intestinal regulatory T cells. Nat. Rev. Immunol. 16, 295–309 (2016).
Cosovanu, C. & Neumann, C. The many functions of Foxp3(+) regulatory T cells in the intestine. Front Immunol. 11, 600973 (2020).
Chapman, P. R., Giacomin, P., Loukas, A. & McCarthy, J. S. Experimental human hookworm infection: a narrative historical review. PLoS Negl. Trop. Dis. 15, e0009908 (2021).
Langenberg, M. C. C. et al. A controlled human Schistosoma mansoni infection model to advance novel drugs, vaccines and diagnostics. Nat. Med 26, 326–332 (2020).
Elliott, D. E. & Weinstock, J. V. Nematodes and human therapeutic trials for inflammatory disease. Parasite Immunol. 39 (2017).
Croese, J. et al. Experimental hookworm infection and gluten microchallenge promote tolerance in celiac disease. J. Allergy Clin. Immunol. 135, 508–516.e5 (2015).
Daveson, A. J. et al. Effect of hookworm infection on wheat challenge in celiac disease – a randomised double-blinded placebo controlled trial. PLoS ONE 6, e17366 (2011).
Büning, J. et al. Helminths as governors of inflammatory bowel disease. Gut 57, 1182–1183 (2008).
Douglas, B. et al. Immune system investigation using parasitic helminths. Annu. Rev. Immunol. 39, 639–665 (2021).
Elliott, D. E. & Weinstock, J. V. Nematodes and human therapeutic trials for inflammatory disease. Parasite Immunol. 39, e12407 (2017).
Loukas, A., Maizels, R. M. & Hotez, P. J. The yin and yang of human soil-transmitted helminth infections. Int. J. Parasitol. 51, 1243–1253 (2021).
Gaze, S. et al. Characterising the mucosal and systemic immune responses to experimental human hookworm infection. PLoS Pathog. 8, e1002520 (2012).
Wright, V. & Bickle, Q. Immune responses following experimental human hookworm infection. Clin. Exp. Immunol. 142, 398–403 (2005).
Mortimer, K. et al. Dose-ranging study for trials of therapeutic infection with Necator americanus in humans. Am. J. Trop. Med. Hyg. 75, 914–920 (2006).
Croese, J., Gaze, S. T. & Loukas, A. Changed gluten immunity in celiac disease by Necator americanus provides new insights into autoimmunity. Int. J. Parasitol. 43, 275–282 (2013).
Diemert, D. et al. Controlled human hookworm infection: accelerating human hookworm vaccine development. Open Forum Infect. Dis. 5, ofy083 (2018).
McSorley, H. J. et al. Suppression of inflammatory immune responses in celiac disease by experimental hookworm infection. PLoS ONE 6, e24092 (2011).
Janse, J. J. et al. Establishing the production of male schistosoma mansoni cercariae for a controlled human infection model. J. Infect. Dis. 218, 1142–1146 (2018).
Langenberg, M. C. C. et al. Katayama syndrome without schistosoma mansoni eggs. Ann. Intern. Med. 170, 732–733 (2019).
Koopman, J. P. et al. Risk assessment for the implementation of controlled human Schistosoma mansoni infection trials in Uganda. AAS Open Res. 2, 17 (2019).
Yordanova, I. A. et al. The worm-specific immune response in multiple sclerosis patients receiving controlled Trichuris suis ova immunotherapy. Life (Basel, Switz.) 11, 101 (2021).
Benzel, F. et al. Immune monitoring of Trichuris suis egg therapy in multiple sclerosis patients. J. Helminthol. 86, 339–347 (2012).
Elliott, D. E., Summers, R. W. & Weinstock, J. V. Helminths and the modulation of mucosal inflammation. Curr. Opin. Gastroenterol. 21, 51–58 (2005).
Sandborn, W. et al. Randomised clinical trial: the safety and tolerability of Trichuris suis ova in patients with Crohn’s disease. Aliment. Pharmacol. Ther. 38, 255–263 (2013).
Summers, R. W., Elliott, D. E. & Weinstock, J. V. Is there a role for helminths in the therapy of inflammatory bowel disease? Nat. Clin. Pract. Gastroenterol. Hepatol. 2, 62–63 (2005).
Summers, R. W. et al. Trichuris suis seems to be safe and possibly effective in the treatment of inflammatory bowel disease. Am. J. Gastroenterol. 98, 2034–2041 (2003).
Billipp, T. E., Nadjsombati, M. S. & von Moltke, J. Tuning tuft cells: new ligands and effector functions reveal tissue-specific function. Curr. Opin. Immunol. 68, 98–106 (2021).
Konnikova, L. et al. High-dimensional immune phenotyping and transcriptional analyses reveal robust recovery of viable human immune and epithelial cells from frozen gastrointestinal tissue. Mucosal Immunol. 11, 1684–1693 (2018).
Devlin, J. C. et al. Single-cell transcriptional survey of ileal-anal pouch immune cells from ulcerative colitis patients. Gastroenterology 160, 1679–1693 (2021).
Matsuzawa-Ishimoto, Y. et al. An intestinal organoid-based platform that recreates susceptibility to T-cell-mediated tissue injury. Blood 135, 2388–2401 (2020).
Corridoni, D., Chapman, T., Antanaviciute, A., Satsangi, J. & Simmons, A. Inflammatory bowel disease through the lens of single-cell RNA-seq technologies. Inflamm. Bowel Dis. 26, 1658–1668 (2020).
James, K. R., Elmentaite, R., Teichmann, S. A. & Hold, G. L. Redefining intestinal immunity with single-cell transcriptomics. Mucosal Immunol. 1–11 (2021).
Fawkner-Corbett, D. et al. Spatiotemporal analysis of human intestinal development at single-cell resolution. Cell 184, 810–826.e23 (2021).
Schreurs, R. et al. Human fetal TNF-alpha-cytokine-producing CD4(+) effector memory T cells promote intestinal development and mediate inflammation early in life. Immunity 50, 462–476.e8 (2019).
Drokhlyansky, E. et al. The human and mouse enteric nervous system at single-cell resolution. Cell 182, 1606–1622.e23 (2020).
Kiner, E. et al. Gut CD4(+) T cell phenotypes are a continuum molded by microbes, not by TH archetypes. Nat. Immunol. 22, 216–228 (2021).
Haber, A. L. et al. A single-cell survey of the small intestinal epithelium. Nature 551, 333–339 (2017).
Duque-Correa, M. A., Maizels, R. M., Grencis, R. K. & Berriman, M. Organoids – new models for host-helminth interactions. Trends Parasitol. 36, 170–181 (2020).
Drurey, C. et al. Intestinal epithelial tuft cell induction is negated by a murine helminth and its secreted products. J. Exp. Med. 219, e20211140 (2022).
Luo, X. C. et al. Infection by the parasitic helminth Trichinella spiralis activates a Tas2r-mediated signaling pathway in intestinal tuft cells. Proc. Natl Acad. Sci. USA 116, 5564–5569 (2019).
Varyani, F. et al. The IL-25-dependent tuft cell circuit driven by intestinal helminths requires macrophage migration inhibitory factor (MIF). Mucosal Immunol. https://doi.org/10.1038/s41385-022-00496-w (2022). Epub ahead of print.
Duque-Correa, M. A. et al. Defining the early stages of intestinal colonisation by whipworms. Nat. Commun. 13, 1725 (2022).
Tuveson, D. & Clevers, H. Cancer modeling meets human organoid technology. Science 364, 952–955 (2019).
Narasimhan, P. B. et al. Similarities and differences between helminth parasites and cancer cell lines in shaping human monocytes: insights into parallel mechanisms of immune evasion. PLoS Negl. Trop. Dis. 12, e0006404 (2018).
Schutgens, F. & Clevers, H. Human organoids: tools for understanding biology and treating diseases. Annu Rev. Pathol. 15, 211–234 (2020).
Neal, J. T. et al. Organoid modeling of the tumor immune microenvironment. Cell 175, 1972–1988.e16 (2018).
Sharpton, T. J., Combrink, L., Arnold, H. K., Gaulke, C. A. & Kent, M. Harnessing the gut microbiome in the fight against anthelminthic drug resistance. Curr. Opin. Microbiol 53, 26–34 (2020).
Chen, H. et al. Dissection of the gut microbiota in mothers and children with chronic Trichuris trichiura infection in Pemba Island, Tanzania. Parasit. Vectors 14, 62 (2021).
Gui, Q. F. et al. Gut microbiota signatures in Schistosoma japonicum infection-induced liver cirrhosis patients: a case-control study. Infect. Dis. Poverty 10, 43 (2021).
Jiang, Y. et al. Alteration of the fecal microbiota in Chinese patients with Schistosoma japonicum infection. Parasite 28, 1 (2021).
Partida-Rodriguez, O. et al. Exposure to parasitic protists and helminths changes the intestinal community structure of bacterial communities in a cohort of mother-child binomials from a semirural setting in Mexico. mSphere 6, e0008321 (2021).
Osakunor, D. N. M. et al. The gut microbiome but not the resistome is associated with urogenital schistosomiasis in preschool-aged children. Commun. Biol. 3, 155 (2020).
Rubel, M. A. et al. Lifestyle and the presence of helminths is associated with gut microbiome composition in Cameroonians. Genome Biol. 21, 122 (2020).
Ajibola, O. et al. Urogenital schistosomiasis is associated with signatures of microbiome dysbiosis in Nigerian adolescents. Sci. Rep. 9, 829 (2019).
Huwe, T. et al. Interactions between parasitic infections and the human gut microbiome in Odisha, India. Am. J. Trop. Med Hyg. 100, 1486–1489 (2019).
Toro-Londono, M. A., Bedoya-Urrego, K., Garcia-Montoya, G. M., Galvan-Diaz, A. L. & Alzate, J. F. Intestinal parasitic infection alters bacterial gut microbiota in children. PeerJ 7, e6200 (2019).
Xu, M. et al. Altered gut microbiota composition in subjects infected with Clonorchis sinensis. Front Microbiol 9, 2292 (2018).
Jenkins, T. P. et al. Infections by human gastrointestinal helminths are associated with changes in faecal microbiota diversity and composition. PLoS ONE 12, e0184719 (2017).
Lee, S. C. et al. Helminth colonization is associated with increased diversity of the gut microbiota. PLoS Negl. Trop. Dis. 8, e2880 (2014).
Gordon, C. A. et al. Helminths, polyparasitism, and the gut microbiome in the Philippines. Int J. Parasitol. 50, 217–225 (2020).
Prommi, A. et al. Intestinal parasites in rural communities in Nan Province, Thailand: changes in bacterial gut microbiota associated with minute intestinal fluke infection. Parasitology 147, 972–984 (2020).
Ramirez-Carrillo, E. et al. Disturbance in human gut microbiota networks by parasites and its implications in the incidence of depression. Sci. Rep. 10, 3680 (2020).
Easton, A. V. et al. The impact of anthelmintic treatment on human gut microbiota based on cross-sectional and pre- and postdeworming comparisons in Western Kenya. mBio 10, e00519–19 (2019).
Lee, S. C. et al. Linking the effects of helminth infection, diet and the gut microbiota with human whole-blood signatures. PLoS Pathog. 15, e1008066 (2019).
Martin, I. et al. The effect of gut microbiome composition on human immune responses: an exploration of interference by helminth infections. Front Genet 10, 1028 (2019).
Jenkins, T. P. et al. A comprehensive analysis of the faecal microbiome and metabolome of Strongyloides stercoralis infected volunteers from a non-endemic area. Sci. Rep. 8, 15651 (2018).
Martin, I. et al. Dynamic changes in human-gut microbiome in relation to a placebo-controlled anthelminthic trial in Indonesia. PLoS Negl. Trop. Dis. 12, e0006620 (2018).
Rosa, B. A. et al. Differential human gut microbiome assemblages during soil-transmitted helminth infections in Indonesia and Liberia. Microbiome 6, 33 (2018).
Schneeberger, P. H. H. et al. Investigations on the interplays between Schistosoma mansoni, praziquantel and the gut microbiome. Parasit. Vectors 11, 168 (2018).
Ramanan, D. et al. Helminth infection promotes colonization resistance via type 2 immunity. Science 352, 608–612 (2016).
Kay, G. L. et al. Differences in the faecal microbiome in Schistosoma haematobium infected children vs. uninfected children. PLoS Negl. Trop. Dis. 9, e0003861 (2015).
Cooper, P. et al. Patent human infections with the whipworm, Trichuris trichiura, are not associated with alterations in the faecal microbiota. PLoS ONE 8, e76573 (2013).
Schneeberger, P. H. H. et al. Different gut microbial communities correlate with efficacy of albendazole-ivermectin against soil-transmitted helminthiases. Nat. Commun. 13, 1063 (2022).
Yang, C. A. et al. Impact of Enterobius vermicularis infection and mebendazole treatment on intestinal microbiota and host immune response. PLoS Negl. Trop. Dis. 11, e0005963 (2017).
Kupritz, J., Angelova, A., Nutman, T. B. & Gazzinelli-Guimaraes, P. H. Helminth-induced human gastrointestinal dysbiosis: a systematic review and meta-analysis reveals insights into altered taxon diversity and microbial gradient collapse. mBio 12, e0289021 (2021).
Gupta, V. K., Paul, S. & Dutta, C. Geography, ethnicity or subsistence-specific variations in human microbiome composition and diversity. Front Microbiol 8, 1162 (2017).
Durazzi, F. et al. Comparison between 16S rRNA and shotgun sequencing data for the taxonomic characterization of the gut microbiota. Sci. Rep. 11, 3030 (2021).
Levy, M., Blacher, E. & Elinav, E. Microbiome, metabolites and host immunity. Curr. Opin. Microbiol 35, 8–15 (2017).
Rooks, M. G. & Garrett, W. S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 16, 341–352 (2016).
Lin, J. D. & Loke, P. Helminth infections and cardiovascular diseases: a role for the microbiota and Mϕs? J. Leukoc. Biol. 110, 1269–1276 (2021).
Rapin, A. & Harris, N. L. Helminth-bacterial interactions: cause and consequence. Trends Immunol. 39, 724–733 (2018).
Cantacessi, C. et al. Impact of experimental hookworm infection on the human gut microbiota. J. Infect. Dis. 210, 1431–1434 (2014).
Ducarmon, Q. R. et al. Dynamics of the bacterial gut microbiota during controlled human infection with Necator americanus larvae. Gut Microbes 12, 1–15 (2020).
Jenkins, T. P. et al. Experimental infection with the hookworm, Necator americanus, is associated with stable gut microbial diversity in human volunteers with relapsing multiple sclerosis. BMC Biol. 19, 74 (2021).
Giacomin, P. et al. Changes in duodenal tissue-associated microbiota following hookworm infection and consecutive gluten challenges in humans with coeliac disease. Sci. Rep. 6, 36797 (2016).
Smallwood, T. B. et al. Helminth immunomodulation in autoimmune disease. Front Immunol. 8, 453 (2017).
Acknowledgements
This work was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, NIH. The authors apologize to those authors whose exemplary work we are unable to cite. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the views of the National Institute of Health. The figure in this manuscript was created with BioRender.com
Author information
Authors and Affiliations
Contributions
P.L., S.C.L., and O.O.O. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Loke, P., Lee, S.C. & Oyesola, O.O. Effects of helminths on the human immune response and the microbiome. Mucosal Immunol 15, 1224–1233 (2022). https://doi.org/10.1038/s41385-022-00532-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41385-022-00532-9
This article is cited by
-
Effect of experimental hookworm infection on insulin resistance in people at risk of type 2 diabetes
Nature Communications (2023)
-
Lessons from helminths: what worms have taught us about mucosal immunology
Mucosal Immunology (2022)
-
Updated immunomodulatory roles of gut flora and microRNAs in inflammatory bowel diseases
Clinical and Experimental Medicine (2022)