Abstract
This study was to explore a novel IL-33/ST2/IL-9/IL-9R signaling pathway that disrupts ocular surface barrier and amplifies allergic inflammation. Two murine models of experimental allergic conjunctivitis (EAC) and IL-9 topical challenge in wild type Balb/c and ST2−/− mice, and two culture models of primarily human corneal epithelial cells (HCECs) and mouse CD4+ T cells were performed. Clinical manifestations, Oregon-Green Dextran (OGD) staining, the apical junction complexes (AJCs), IL-33/ST2 and IL-9/IL-9R signaling molecules were evaluated in ocular surface and its draining cervical lymph nodes (CLNs) by RT-qPCR, immunostaining and ELISA. The typical allergic signs, enhanced OGD staining intensity, disrupted morphology of AJCs, including ZO-1, claudin 1, occludin, and E-cadherin, and the stimulated signaling of IL-33/ST2 and IL-9/IL-9R were observed in ocular mucosa and draining CLNs in EAC-Balb/c mice, but significantly reduced or eliminated in EAC-ST2−/− mice. Topical challenge of IL-9 resulted in the obvious OGD staining and disrupted ocular surface AJCs in Balb/c mice and in HCECs in vitro. IL-9 production was found to be stimulated by IL-33 in CD4+ cells from Balb/c mice in vitro. Our findings uncovered a novel phenomenon and mechanism by which ocular surface barrier integrity is disrupted in allergic conjunctivitis by IL-33/ST2/IL-9/IL-9R signaling pathway, which may amplify the allergic inflammation.
Similar content being viewed by others
Introduction
Allergic diseases like asthma, atopic dermatitis, and seasonal allergy, affect large populations worldwide with up to 50% of these individuals reporting ocular allergic manifestations.1,2,3 One of the major pathogenic mechanism of allergic inflammation has been attributed to Th2-dominant hypersensitivity.4 Emerging studies have revealed novel cellular and molecular mechanisms by which the epithelium modulates Th2 responses through producing pro-allergic cytokines, including interleukin (IL)-335 and thymic stromal lymphopoietin (TSLP).6 Allergic disease is increasingly being seen as an epithelial disease both structurally and functionally. In addition, an intact mucosal epithelial barrier is considered to be crucial for the maintenance of ocular surface steady as it protects the host immune system from exposure to allergens and noxious environmental triggers.7 Compelling evidence demonstrates that the epithelial barrier dysfunction plays vital role in developing allergic inflammation in the airways,8 skin,9 and gut.10 However, it remains to be elucidated what molecular mechanisms and pathways drive barrier dysfunction. The ocular surface is the most common site for developing allergic inflammation, because allergens can easily deposit directly onto the exposed mucosal surface of the eye. The precise pathogenesis of barrier dysfunction in patients with allergic conjunctivitis needs to be further explored.
In the ocular surface, corneal and conjunctival epithelial cells form a barrier to the outside world, which termed apical junctional complexes (AJCs) that form between neighboring cells.11 AJCs consist of the apical tight junctions (TJs) and underlying adheren junctions (AJs) that bind together through homotypic and heterotypic interactions, which not only adjust cell–cell contact but also construct cell polarity, and regulate the paracellular movement of ions and macromolecules. TJ complexes are composed of transmembrane proteins, such as all kinds of claudins (claudin 1 is the primary claudin protein expressed by ocular surface) and occludin, and membrane-associated proteins such as zonula occludens (ZO)-1, -2, and -3. AJs are essential for tethering forces generated between the adjacent cells. AJs consist of a transmembrane protein (epithelial cadherin, or E-cadherin) and cytoplasmic linker proteins (catenin α, β, γ). The cytoplasmic domains of the transmembrane molecules in the TJs and AJs are structurally and functionally linked to the thick cytoskeletal cortical filamentous actin band via linker proteins.
Recent studies have documented the presence of dysfunctional epithelial AJCs in the airways and gut of allergic diseases. Airway epithelial brushings from patients with asthma have significantly lower claudin 18 than those from healthy controls. The loss of claudin 18 was sufficient to impair epithelial barrier function in the airways of claudin 18 null mouse.12 In murine model of experimental colitis, the abundance of the pore-forming factor claudin 2, which is known to impair barrier function, was observed to be higher, while the abundance of the sealing factor claudin 3 and tight junction protein occludin were found to be lower than those in the untreated (UT) control mice. The translocation of bacteria in the mucosa increased significantly in the experimental colitis mice when compared with controls.13 In the differentiated primary sinonasal epithelial cells, the exposure to IL-4 and IL-13 resulted in the decreased expression of TJ protein JAM-A and AJ protein E-cadherin with decreased transepithelial resistance.14 However, the mechanisms of detective epithelial AJCs in the allergic response are only partly known and need to be further elaborated.
Th9 cells is recent identified a subset of T helper cells characterized by producing IL-9, which are developed from native T cells under the influence of IL-4.15 Recent studies suggest that IL-9/IL-9R signaling pathway is activated in allergic diseases. There was a significant increase in the expression of IL-9 or its receptor IL-9R in asthmatic airways compared with chronic bronchitis, sarcoidosis and healthy control subjects.16 IL-9 expression was increased after segmental allergen challenge in allergic asthmatics.17 Recently, IL-9-secreting Th9 cells are known to be involved in the pathogenesis of inflammatory bowel disease.18 Furthermore, IL-9 has been demonstrated to be increased significantly in the sera of rheumatoid and psoriatic arthritis, systemic vasculitis, systemic lupus erythematosus and systemic sclerosis patients.19 Statistically significantly higher expression of IL-9 was observed in the supernatants of cultured peripheral blood mononuclear cells and CD4+ T cells from patients with active Vogt–Koyanagi–Harada disease compared with that in cells from normal controls.20
However, the novel mechanism by which IL-9/IL-9R signaling pathway involves in the disruption of epithelial barrier in Th2-dominant allergic inflammation is largely unknown. The present study was conducted to uncover whether ocular surface mucosal epithelium suffers barrier disruption, and what molecular signaling pathway causes the disruption of mucosal barrier integrity in allergic disease using a well-characterized murine model of allergic conjunctivitis in wild-type (WT) Balb/c and ST2 knockout (ST2−/−) mice, a murine topical ocular surface challenge model, and a culture model of primary human corneal epithelial cells (HCECs) exposed to IL-9.
Results
The integrity of ocular surface epithelial barrier was disrupted in murine experimental allergic conjunctivitis (EAC) model
To evaluate the integrity of ocular surface epithelial barrier in allergic inflammation, a murine model of EAC was induced in Balb/c mice sensitized and topically challenged by SRW pollen, with phosphate buffered saline (PBS)-treated and UT mice as controls. Repeated topical challenges with SRW allergen generated typical signs mimic to human allergic conjunctivitis, including lid edema, conjunctival redness, chemosis, tearing, unsmooth cornea, and frequent scratching of the eye lids (Fig. 1a).
EAC model was induced in Balb/c mice sensitized and topically challenged for 7 days by SRW pollen, with PBS-treated and UT mice as control groups. The clinical signs were detected through the slit lamp microscope (a). OGD staining of cornea in EAC mice was carried out and scored (**P < 0.01, compared with UT control) (b, c). AJCs structure proteins, including ZO-1, claudin 1, occludin, and E-cadherin, were detected in the corneal and conjunctival epithelial cells through whole-mount IF staining with quantification by ImageJ (d, e). Results shown are the mean ± SD (n = 5). *P < 0.05, **P < 0.01, ***P < 0.001, compared with PBS controls. Bar: 20 μm.
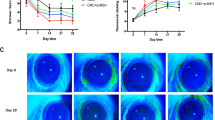
To assess corneal epithelial barrier integrity, Oregon-Green Dextran (OGD) staining was performed. Compared with PBS and UT mice, corneal uptake of OGD increased in the EAC mice. There were many widespread punctuate and confluent staining on EAC mouse corneas (Fig. 1b), suggesting corneal epithelial barrier disrupted with some cell loss. The OGD intensity score, used to measure the corneal surface disrupted areas, showed that the average OGD intensity score in eyes of EAC group were 755.0 ± 57.98 (P < 0.01), dramatically higher than UT (213.2 ± 13.24) or PBS (261.4 ± 28.84) group (Fig. 1c).
To further evaluate the integrity of ocular surface epithelial barrier, immunofluorescent (IF) staining was performed to evaluate the integrity of major TJ and AJ proteins, including ZO-1, claudin 1, occludin, and E-cadherin. As shown in Fig. 1d, the immunoreactivities of ZO-1, claudin 1, occludin, and E-cadherin were displayed contiguously at the epithelial cell borders that form an integral net structure on the cornea and conjunctiva of the PBS mice. However, these barrier proteins were significantly disrupted by showing their discontinuous and incomplete staining in cell boundaries on the corneal and conjunctival epithelia in EAC mice. The quantitative analysis clearly showed the disrupted barrier structure in EAC mice by measuring the IF length of each apical junction protein on the images (Fig. 1e).
Increased IL-9/IL-9R signaling was observed in EAC mice
Th9 cells that secret IL-9 has been known to play an important role in allergic diseases.21,22,23 In addition to Th2-dominant condition, Th9 cells were observed to increase significantly in the EAC mice. As evaluated by real-time quantitative polymerase chain reaction (RT-qPCR) (Fig. 2a), mRNA expression of IL-9 was dramatically upregulated in the conjunctival tissue (10.91 ± 2.58-fold, P < 0.01) of EAC mice when compared with PBS control group. IL-9 protein levels (Fig. 2b) in conjunctival cells, measured by enzyme-linked immunosorbent assay (ELISA), increased significantly to 92.33 ± 13.20 pg/mg in EAC mice, 2.54 fold higher than that in PBS controls (36.33 ± 8.50 pg/mg, P < 0.01, Fig. 2b). These results suggest that Th9 cells were infiltrated in the ocular conjunctival tissue in EAC mice. Interestingly, in the ocular draining cervical lymph nodes (CLNs) of EAC mice, IL-9 was upregulated significantly at mRNA levels (8.18 ± 1.08-fold, P < 0.05) and also at protein levels, which was 120.12 ± 10.03 pg/mg, 2.17-fold higher than that 55.41 ± 9.06 pg/mg (P < 0.05) in PBS control mice (Fig. 2a, b). IF double staining further showed that the ocular mucosal tissue was infiltrated by many Th9 cells that express IL-9 and T help cells marker CD4+ in the ocular mucosa; and these double positive Th9 cells also largely increased in the draining CLNs in EAC mice when compared with those in PBS mice (Fig. 2c). These findings indicated that the Th9 cell-secreted IL-9 signaling was largely stimulated in ocular mucosa and draining CLNs in the EAC mice.
In conjunctiva (Conj), cornea and/or CLN of PBS-treated mice and EAC mice, IL-9 (a), IL-9R (d) and related signal pathway molecules (SPI 1, NFATc1, and NFATc2) (f) mRNA expression were evaluated by RT-qPCR; IL-9 production was detected by ELISA (b). In PBS-treated mice and EAC mice, CD4 and IL-9 expression in Conj and CLN (c) were shown by IF double staining; IL-9R expression in cornea and Conj was shown by IHC staining (e); the expression of PU.1, NFATc1, and NFATc2 in CLN were shown by IF staining (g). Results shown are the mean ± SD (n = 5). *P < 0.05, **P < 0.01, ***P < 0.001, compared with PBS controls. Bar: 20 μm.
Interestingly, the expression of IL-9R by ocular epithelial cells increased markedly in EAC mice. RT-qPCR results showed that IL-9R mRNA expression was significantly upregulated in the conjunctival (2.84 ± 0.26-fold, P < 0.05) and corneal (6.10 ± 0.28-fold, P < 0.01) epithelia in EAC mice compared with PBS controls (Fig. 2d). The immunohistochemical (IHC) staining confirmed that IL-9R protein was weakly positive by normal corneal and conjunctival epithelial cells in PBS control mice, but the IL-9R immunoreactivity increased significantly by ocular surface, especially in basal and suprabasal layers of conjunctival epithelial cells in EAC mice (Fig. 2e). These findings indicated the increase of IL-9/IL-9R signaling in the EAC model.
PU.1 (encoded by SPI 1),24 NFATc1,25 and NFATc226 are critical factors in diverting T cells into an IL-9-secreting lineage and regulates the development of Th9 cells. To reveal the activation of IL-9 signaling in EAC mice, the expression of PU.1, NFATc1, and NFATc2 was evaluated. By RT-qPCR, mRNA expression of SPI 1, NFATc1, and NFATc2 were significantly upregulated in the conjunctival (2.05 ± 0.08, P < 0.05; 4.54 ± 0.98, P < 0.01; and 5.80 ± 0.06-fold, P < 0.001, respectively) and CLNs (2.38 ± 0.04, 2.25 ± 0.09, 2.39 ± 0.18-fold, respectively, all P < 0.05) of EAC mice, compared with PBS controls (Fig. 2f). By IF staining, the positive cells of PU.1, NFATc1, and NFATc2 increased in CLNs of EAC mice, while these proteins were almost absent in CLNs of PBS controls (Fig. 2g). These results supported our finding that IL-9/IL-9R signaling was stimulated in EAC model.
The disruptive effects of IL-9 on ocular surface epithelial barrier in Balb/c mice in vivo and in primary HCECs in vitro
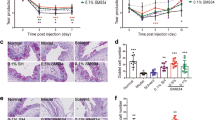
To explore whether IL-9 is a responsive factor involved in disruption of ocular surface barrier, we created a topical challenge murine model treated with recombinant mouse IL-9 (rmIL-9) at 1–50 ng/ml, 10 μl/eye, three times each day for 3 days. The topical challenges with rmIL-9 resulted in unsmooth cornea through slit lamp microscopy (Fig. 3a), and a significant increase in corneal permeability measured by OGD staining (Fig. 3b). OGD intensity scores were dramatically increased at a dose-dependent manner to 385.4 ± 169.49 (P > 0.05), 1202.4 ± 384.77 (P < 0.01), and 2262.0 ± 556.26 (P < 0.001) fold in mouse corneas exposed to rmIL-9 at 1, 10, and 50 ng/ml, respectively, compare to PBS controls (242.8 ± 72.92) (Fig. 3c).
The ocular surface of Balb/c mice was topically challenged by different concentration (0–50 ng/ml) rmIL-9 three times each day for 3 days. The ocular surface clinical signs were observed through the slit lamp microscope (a). OGD staining in the cornea was carried out and scored (**P < 0.01; ***P < 0.001, compared with PBS control) (b, c). ZO-1, claudin 1, occludin, and E-cadherin were detected in the corneal and conjunctival epithelial cells through whole-mount IF staining with quantification (d, e). The structures of ZO-1, claudin 1, occludin, and E-cadherin were shown in HCECs treated by 10 ng/ml rhIL-9 for 48 h (f, g). Results shown are the mean ± SD (n = 5). *P < 0.05, **P < 0.01, ***P < 0.001, compared with PBS controls. Bar: 20 μm.
As shown in Fig. 3d, e by IF staining with quantification, the protein expression of ZO-1, claudin 1, occludin, and E-cadherin was intact and complete with an intricate 3D net morphology in corneal and conjunctival epithelia of the PBS control mice. However, the abundance and integrity of these TJs and of E-cadherin was low and partially lost with the disrupted morphology in corneal and conjunctival epithelia of the rmIL-9-treated mice, which suggest that the disruptive effects of IL-9 on epithelial barrier in vivo.
To further confirm the effects of IL-9 on epithelial barrier, primary HCECs were cultured from normal human corneal tissues and treated with 10 ng/ml of recombinant human IL-9 (rhIL-9) for 48 h in vitro. As shown in Fig. 3f, g, ZO-1, claudin 1, occludin, and E-cadherin were continuously expressed to form intact cell borders in UT condition, but their immunoreactivities showed largely disrupted integrity with incomplete net morphology in IL-9-treated cultures.
IL-9 overproduction was induced by epithelium-derived IL-33 in allergic inflammation
Our previous study have shown that IL-33/ST2 signaling pathway activation in EAC mice.27 In the present study, IL-33 mRNA expression was significantly upregulated in the conjunctival (3.31 ± 0.26-fold) and corneal (3.65 ± 0.11-fold) epithelia (all P < 0.01) from EAC mice compared with PBS controls (Fig. 4a). IL-33 protein production quantified by ELISA was also dramatically elevated in the conjunctival epithelia by 2.55 fold (from 480.67 ± 30.25 to 1223.33 ± 150.44 pg/mg, P < 0.05) from EAC mice compared with PBS mice (Fig. 4b). IL-33 immunoreactivity was weak in the corneal and conjunctival epithelia of PBS mice. Significant elevated IL-33 expression was observed in the corneal and conjunctival epithelia of EAC mice (Fig. 4c). As receptor of IL-33, ST2 is mainly expressed by Th2 cells28,29 and dendritic cells.30,31 The mRNA of membrane bound ST2 was largely upregulated in the conjunctival tissue (2.98 ± 0.21-fold, P < 0.01) and CLNs (2.33 ± 0.39-fold, P < 0.05) of EAC mice compared with PBS controls (Fig. 4d). IF double staining showed the increase of CD4+ T cells, some of which produce ST2 protein, in the conjunctival stroma of EAC mice, indicating the infiltrated Th2 cells in ocular surface of EAC mice. The CD4+ST2+ cells were also found to increase markedly in CLNs of EAC mice when compared with PBS control mice (Fig. 4e).
In conjunctiva (Conj), cornea and/or CLN of PBS-treated mice and EAC mice, IL-33 (a) and ST2 (d) mRNA expressions were evaluated by RT-qPCR; IL-33 production was detected by ELISA (b). In PBS-treated mice and EAC mice, IL-33 expression in cornea and Conj was shown by IHC staining (c); ST2 and CD4 expression in Conj and CLN (e) were shown by IF double staining. Direct effects of rmIL-33 on IL-9 expression in isolated mice CD4+ T cells was detected at mRNA (f, up) and protein (f, down) levels. IL-9 signal pathway related proteins (including PU.1, NFATc1, and NFATc2) were evaluated in mice CD4+ T cells induced by rmIL-33 (g). Results shown are the mean ± SD (n = 5), *P < 0.05, **P < 0.01, compared with PBS controls. Bar: 20 μm.
To investigate the direct effects of IL-33 on IL-9, CD4+ T cells were isolated from CLNs and spleens of Balb/c mice, and treated with 10 ng/ml recombinant mouse IL-33 (rmIL-33) for different period (6–48 h). As shown in Fig. 4f, IL-9 mRNA expression was time-dependently induced by rmIL-33 for 6–48 h (1.45 ± 0.17, P < 0.01; 4.28 ± 0.09, P < 0.01; and 39.98 ± 6.36-fold, P < 0.01, respectively) while IL-9 mRNA was barely detectable in UT group. ELISA data confirmed these inducible effects of IL-33 on IL-9 production at protein levels. The protein production of IL-9 from UT control (no detected expression) significantly increased to (7.33 ± 4.16 pg/ml, p > 0.05; and 38.05 ± 14.02 pg/ml, p < 0.05, respectively) in the culture media of T cells treated by rmIL-33 for 24 and 48 h. At UT culture, the expression of PU.1, NFATc1, and NFATc2 were nearly negative by IF staining. However, these proteins were observed to markedly increase in rmIL-33-treated CD4+ T cells for 48 h (Fig. 4g). These findings demonstrate that IL-33 induces the expression and production of IL-9 by CD4+ T cells.
IL-33/ST2/IL-9/IL-9R signaling was involved in disruption of ocular surface epithelial barrier in allergic conjunctivitis
It has not been documented that epithelial-derived pro-allergic cytokine IL-33 disrupts the barrier of corneal and conjunctival epithelia integrity in allergic inflammation through activation of ST2 receptor on T help cells to produce IL-9, which binds to IL-9R on ocular surface epithelial cells. To confirm this notion, ST2−/− mice were used to create EAC model with the same protocol with WT Balb/c mice. As shown previously, repeated topical challenges with SRW allergen generated typical signs mimic to human allergic conjunctivitis in Balb/c mice. However, these clinical signs were not displayed in EAC model in ST2−/− mice (Fig. 5a). Th2 cytokines IL-4, IL-5, and IL-13, shown by RT-qPCR (Fig. 5b) and ELISA (Fig. 5c), were significantly induced in mRNA and protein levels in corneal and conjunctiva epithelium and CLNs in WT Balb/c mice, but not in ST2−/− mice. The corneal permeability measured by OGD staining between UT, PBS, and EAC group had no significant change in ST2−/− mice (Fig. 5d, e). As well as the structure of ZO-1, claudin 1, occludin, and E-cadherin in corneal and conjunctival epithelial cells in EAC model of ST2−/− mice was still complete, similar with PBS model of ST2−/− mice (Fig. 5f, g). These findings suggest that ocular surface epithelial cells barrier integrity was disrupted through IL-33/ST2-dependent innate immune response in EAC mice.
EAC model was induced in ST2−/− mice using the same method. The ocular surface clinical signs were observed through the slit lamp microscope (a). Th2 cytokines (IL-4, IL-5, and IL-13) mRNA expressions were evaluated by RT-qPCR in the conjunctiva (Conj), cornea and CLN in EAC-Balb/c mice and EAC-ST2−/− mice, compared with PBS control (b). The protein of Th2 cytokines (IL-4, IL-5, and IL-13) in the conjunctiva (Conj) and CLN of EAC-Balb/c mice and EAC-ST2−/− mice, compared with PBS group (c). OGD staining in the cornea was carried out and scored in EAC model of ST2−/− mice (d, e). ZO-1, claudin 1, occludin, and E-cadherin were detected in the corneal and conjunctival epithelial cells in EAC model and PBS control in ST2−/− mice through whole-mount IF staining with quantification (f. g). Results shown are the mean ± SD (n = 5). *P < 0.05; **P < 0.01, ***P < 0.001, compared with PBS controls. Bar: 20 μm.
In addition, in EAC model of ST2−/− mice compare with PBS group, IL-9 (Fig. 6a–c), IL-9R (Fig. 6d, e) and IL-9 signaling pathway (SPI 1 or PU.1, NFATc1 and NFATc2) (Fig. 6f, g), were not induced at mRNA and protein levels in corneal and conjunctiva epithelium and/or CLNs. These results confirmed that IL-9 induction and IL-9 related signaling activation were initiated through IL-33/ST2-dependent innate immune response in EAC mice.
In ST2−/− mice, IL-9 (a), IL-9R (d), SPI 1, NFATc1, and NFATc2 (f) mRNA expression in the conjunctiva (Conj), cornea and/or CLN of EAC model was evaluated by RT-PCR. IL-9 protein expression in the conjunctiva (Conj) and CLN of EAC mice was detected by ELISA (b) and IF staining (c). IL-9R protein expression in the ocular surface (e) and PU.1, NFATc1 NFATc2 protein expression in CLN (g) were shown by IHC staining and IF staining respectively. Results shown are the mean ± SD (n = 5). Bar: 20 μm.
Finally, we performed a topical challenge experiment with rmIL-9 in ST2−/− mice. Interestingly, the topical administration of rmIL-9 caused ocular barrier disruption in ST2−/− mice. As shown in Fig. 7a, the rmIL-9 topical challenges resulted in a significant increase in corneal epithelial defects when compared with PBS-treated controls, as detected by OGD staining. As shown in Fig. 7b–d, IF staining displayed the intact and complete morphology and structure of ZO-1, claudin 1, occludin, and E-cadherin in corneal and conjunctival epithelia of the PBS-controlled ST2−/− mice. However, the integrity of these barrier proteins was significantly disrupted in the rmIL-9-treated ST2−/− mice. The results further confirmed that IL-9 signaling is a causative downstream pathway of IL-33/ST2.
The ocular surface of ST2−/− mice was topically administrated by rmIL-9 at 50 ng/ml, three times per day for 3 days, with PBS eyedrop as control. a OGD staining in the cornea from ST2−/− mice, which were untreated, treated with PBS or rmIL-9. ZO-1, claudin 1, occludin, and E-cadherin in the corneal (b) and conjunctival (c) epithelia of ST2−/− mice treated with rmIL-9 or PBS control were detected by whole-mount IF staining with quantification (d). Results shown are the mean ± SD (n = 5). *P < 0.05, **P < 0.01, ***P < 0.001, compared with PBS controls. Bar: 20 μm.
Discussion
The barrier function disruption in allergic inflammation has been recognized. Recent studies have revealed that epidermal barrier dysfunction plays a major role in allergen sensitization and development of atopic dermatitis, which ignited the inception of allergy march.32 The impairment of airway epithelial barrier was found to be a common feature of asthma, which has been postulated to contribute to immune cell activation and airway hyper-responsiveness.33 In inflammatory bowel diseases, such as Crohn’s disease and ulcerative colitis, the intestinal barrier function was impaired, and mucosal wound healing was suppressed.34 It has been accepted that the epithelial barrier dysfunction and increased permeability may contribute to antigen sensitization and disease progression in allergic disease.8,9
However, the role and cellular and molecular mechanisms of the epithelial barrier disruption in ocular surface allergic diseases have not been well investigated. In the present study, we uncovered a novel phenomenon and mechanism by which IL-33/ST2/IL-9/IL-9R signaling pathway disrupt the corneal and conjunctival epithelial barrier in a murine model of allergic conjunctivitis. We revealed the disruption of corneal and conjunctival epithelial integrity, identified a role of IL-9 in disrupting the ocular barrier by interaction with IL-9R on epithelial cells, and further demonstrated the IL-33/ST2 signaling inducing the IL-9 expression by CD4+ T cells in vivo and in vitro, which exacerbated allergic information.
First, we observed the widespread punctuate damage on corneal surface by OGD staining, and revealed the incomplete and disrupted morphology of the TJ and AJ proteins, ZO-1, claudin 1, occludin, and E-cadherin at cell boundaries of corneal and conjunctival epithelia by immunostaining in EAC model of WT mice, which indicates that the ocular surface barrier integrity was disrupted in allergic inflammation. Second, we revealed that the mucosa barrier structure was impaired directly by IL-9 in vivo and in vitro. With a murine model, the topical challenge of rmIL-9 protein resulted in the significant disruption of ocular surface epithelial barrier showing the punctuate and confluent OGD staining, and the discontinuous morphological IF staining of ZO-1, claudin 1, occludin, and E-cadherin, which are similar to that observed in EAC mice. Using primary HCECs cultures, we further confirmed that IL-9 exposure led to the morphological and structural disruption of these TJ and AJ proteins in vitro.
Our previous study had shown that IL-33 expression was largely increased in the corneal and conjunctival epithelial cells in EAC mice.27 IL-33 mediates its function through its interaction with receptor complex consisting of ST2 membrane bound (ST2L)28 molecule and IL-1R accessory protein. The present study further confirmed that IL-33/ST2 signaling is involved in inducing IL-9 production in EAC mice, but IL-9 upregulation and barrier disruption were largely reversed in ST2−/− mice. Interestingly, the topical administration of rmIL-9 to ST2−/− mice leads barrier disruption of corneal and conjunctival epithelium as shown in Fig. 7. The results further confirm that IL-9 is a causative downstream signaling pathway of IL-33/ST2 in allergic conjunctivitis.
IL-9 is mainly produced by CD4+ T cells, referred to as Th9, although IL-9 is also expressed by other cells including NK, Mast, and ILC2 cells.35 Here we showed that IL-9 production at mRNA and protein levels were directly induced by IL-33 in the cultured CD4+ T cells isolated from Balb/c mice in vitro. The finding is supported by a previous report that IL-33 can initiate IL-9 protein secretion in vitro in human CD4+ T cells and basophils isolated from peripheral blood.36 Th9 cells, characterized by producing IL-9, are developed from native T cells under the influence of IL-415 and regulated by specific transcription factors, including PU.1 (encoded by SPI 1)24 and NFATc125 and NFATc2.26 Our data also showed the stimulated mRNA expression of SPI 1 and NFATc1 and NFATc2 in EAC mice and in CD4+ T cell cultures exposed to IL-33.
In this study, OGD was used to detect the epithelial barrier disruption in EAC mice. Sodium fluorescein is regularly used in the clinic to evaluate corneal epitheliopathy in patients, while OGD dye has been shown to be more discriminative than fluorescein in mice.37 Small molecule fluorescein (376 MW) diffuses rapidly passing through the murine corneal epithelium into stroma while larger molecule OGD (70,000 MW) is retained at the surface. In an allergic eye disease model by Reyes,38 ~10% of mouse eyes presented with corneal fluorescein staining. The difference in sensitivity of staining could cause the discrepancy seen in the frequency of epitheliopathy between the two models. In the IF images, the barrier disruption was shown mainly as discontinuous and incomplete membrane type of cell junction protein. Several staining appeared partially cytoplasmic in the conjunctiva of the EAC model because these cells were severely disrupted with degraded junction proteins.
IL-9 systems are shown to be important for the pathology of inflammatory diseases, such as colitis, atopic diseases, and inflammatory bowel diseases.9,13 In the colitis murine model,13,34 the increased claudin 2, and decreased claudin 3, and occludin were observed in intestinal epithelial cells of WT mice, but these changes were largely reversed in IL-9 deficient mice, which suggests that IL-9 is involved in intestinal barrier disruption in colitis mice. The pathological role of IL-9 in our EAC model appears relatively comparative to colitis at least in part on the mucosa barrier function.
IL-9 was also found to reduce the epithelial resistance in stratified primary human esophageal epithelial cells, and decrease the membrane bound E-cadherin in epithelial monolayers.39 These findings indicate that IL-9 controls the expression of barrier proteins and barrier function in allergic diseases. IL-9 is also known to induce epithelial hyperplasia in several inflammatory and allergy conditions in various organ systems.40 Future studies are important to explore pathological roles and mechanisms of IL-9 in multiple disease models.
In conclusion, we have uncovered a novel phenomenon and molecular mechanism by which ocular surface barrier integrity was disrupted in allergic conjunctivitis. We show that epithelial-derived pro-allergic cytokine IL-33 is highly stimulated in EAC mice, and IL-33 induced the production IL-9 in CD4+ T cells through ST2 receptor, then IL-9 impaired ocular surface barrier integrity by binding with IL-9R expressed in the corneal and conjunctival epithelial cells. These findings demonstrate that IL-33/ST2/IL-9/IL-9R signaling, as one of IL-33/ST2 pathways, mediates the ocular epithelial barrier in allergic inflammation, and provides potential therapeutic significance in preventing and treating the allergic disease.
Methods
Animals
The animal research protocol was approved by the Institutional Animal Care and Use Committee at Baylor College of Medicine. All animals used in this study were maintained in specific pathogen-free conditions in microisolator cages and were treated in accordance with the guidelines provided in the Association for Research in Vision and Ophthalmology statement for the Use of Animals in Ophthalmic and Vision Research. Balb/c mice aged at 6–8 weeks were purchased from the Jackson Laboratory (Bar Harbor, ME). The homozygote ST2−/− mice in Balb/c background were kindly provided by Dr Andrew N.J. McKenzie, FMedSci, Division of Protein and Nucleic Acid Chemistry, MRC Laboratory of Molecular Biology, Cambridge, UK through Dr Stephanie Cormier at the University of Tennessee, USA. The age- and gender-matched Balb/c and ST2−/− mice grown to 8–10 week old were used for experiments.
Murine model of EAC
The mouse EAC model was induced using the previously reported methods41,42,43 with modification.44 In brief, mice (Balb/c and ST2−/−) were immunized with 50 μg of SRW pollen (Greer Laboratories, Lenoir, NC) in 5 mg of ImjectAlum (Pierce Biotechnology, Rockford, IL) by footpad injection on day 0. Allergic conjunctivitis was induced by repeated topical challenges of 1.5 mg of SRW pollen suspended in 10 ml of PBS, pH 7.2, into each eye once a day from days 10 to 16. PBS eyedrop-treated SRW sensitized and UT mice were used as controls. Animals were examined clinically for signs of immediate hypersensitivity responses 20 min after each topical challenge with SRW pollen. On day 17, 24 h after the last SRW challenge, the corneal barrier integrity in mice was assessed with corneal OGD staining and whole-mount staining. The corneal epithelium, conjunctiva, CLNs and whole eyes were harvested for gene expression assays, ELISA and histopathological studies. Five mice per group were used in each experiment, and the same experiments were repeated five times.
Murine model for ocular surface barrier disruption by IL-9 topical challenge
Wild type Balb/c and ST2−/− mice were topically challenged with rmIL-9 (ProSpec Bio, East Brunswick, NJ) at different concentration (1–50 ng/ml, 10 μl/eye) or 10 μl 0.1% bovine serum albumin (BSA)-PBS as controls three times each day for 3 days. Animals were examined clinically for signs of immediate hypersensitivity responses 20 min after last IL-9 topical challenge each day. Disruptive effect of IL-9 to the corneal barrier integrity was assessed on corneal staining with OGD. Tissue specimens including corneal and conjunctival tissues were fixed in acetone for whole-mount staining. Five mice per group were used in each experiment.
In vitro culture model for human corneal epithelial barrier disruption exposed to IL-9
Fresh human corneoscleral tissues (<72 h after death) from donors aged 35–57 years without allergic conditions were obtained from the Lions Eye Bank of Texas (Houston, TX). HCECs were cultured in eight-well plates using explants from corneal limbal rims in a supplemented hormonal epidermal medium (SHEM) containing 5% fetal bovine serum (FBS) using our previous methods.45 Confluent corneal epithelial cultures were switched to serum-free SHEM and treated for 48 h with 10 ng/ml rhIL-9 (ProSpec Bio, East Brunswick, NJ). Each experiment was repeated five times.
In vitro culture model for direct effects of IL-33 on murine CD4+ cells
Murine CD4+ T cells were isolated from the spleens and CLNs of 6–8-week-old Balb/c mice as described previously.46 In brief, the spleens and CLNs of mice were surgically excised, crushed between two sterile frosted glass slides to make a single cell suspension while red blood cells were lysed with ammonium chloride. The cell suspension was centrifuged, filtered and resuspended with rat anti-mouse CD4-conjugated magnetic microbeads (BD Pharmingen, San Jose, CA) diluted with cold 0.5% BSA-PBS. After incubation for 15 min at 4 °C, the cells were suspended and loaded onto a micro-column. Positive cells attached to the column were removed with a plunger and CD4-enriched cell suspensions contained >80% CD4+ T cells as determined by flow cytometry. The freshly isolated murine CD4+ T cells were cultured in 8 or 24-well plates in RPMI 1640 with 10% FBS and treated for 6–48 h with 10 ng/ml of rmIL-33 (Biolegend, San Diego, CA). Medium supernatant was corrected and CD4+ T cells were lysed for total RNA extraction or cellular proteins. And cultured CD4+ T cells were fixed in acetone for staining of PU.1, NFATc1, and NFATc2.
Corneal staining with Oregon-Green Dextran_488 conjugated dye (OGD)
Corneal epithelial staining with OGD (70,000 MW; Invitrogen-Molecular Probes, Eugene, OR) was assessed in the three different groups37,47 Briefly, 0.5 μl of 50 μg/ml OGD was instilled in the ocular surface 1 min before euthanasia. Eyes were rinsed with 2 ml balanced salt solution and excess liquid was blotted carefully from the ocular surface with filter papers without touching the cornea. Digital pictures of both eyes were taken under 470 nm excitation and 488 nm emission wave lengths using a Nikon (Tokyo, Japan) SMZ-1500 stereo microscope, with an exposure time of 2 s. The mean intensity in the central cornea was evaluated from digital images using Nikon’s universal software (NIS) Elements (version 3.0) by placing a fixed region of interest (a 2-mm diameter circle) on the central cornea. This fluorescence measurement in the central ring was done independently by two masked observers, for each mouse eye.
Total RNA extraction, reverse transcription (RT), and RT-qPCR
Total RNA was isolated from mouse tissue specimens and cultured cells using a Qiagen RNeasy Micro kit according to manufacturer’s protocol, and quantified by NanoDrop® ND-2000 Spectrophotometer and stored at −80 °C. The first-strand cDNA was synthesized by RT from 1.0 μg of total RNA using Ready-To-Go You-Prime First-Strand Beads as previously described.48 RT-qPCR was performed with specific primers and probes using TaqMan® gene expression assays and TaqMan® master mix (Applied Biosystems, Foster City, CA) in StepOnePlus™ Real-Time PCR System (Applied Biosystems, Foster City, CA). TaqMan gene expression assays used for this study were: mouse GAPDH (Mm99999915_g1), IL-9 (Mm00434305_m1), IL-9R (Mm00434313_m1), SPI 1 (Mm00488140_m1), NFATc1 (Mm00479445_m1), NFATc2 (Mm00477776_m1), IL-33 (Mm00505403_m1), ST2 (Mm01233982_m1), IL-4 (Mm00445259_m1), IL-5 (Mm0099999063_m1), and IL-13 (Mm00434204_m1). The results were analyzed by the comparative threshold cycle method and normalized by GAPDH as previously reported.
ELISA
Double-sandwich ELISA for mouse IL-9, IL-33, IL-4, IL-5, and IL-13 was performed to determine their protein levels in the cell lysates from mouse conjunctiva or CLNs, as well as in supernatants of cultured CD4+ T cells, according to the manufacturer’s protocols similar to our previous report.49 Absorbance was read at 450 nm with a reference wavelength of 570 nm by Infinite M200 microplate reader (Tecan US, Inc., Morrisville, NC). Mouse ELISA kits for IL-9, IL-33, IL-4, and IL-5 were from Biolegend (San Diego, CA). Mouse ELISA kit for IL-13 was from R&D Systems™ (Minneapolis, MN).
IF and IHC staining
The eyes and lids or CLNs of mice in each group were excised, embedded in optimal cutting temperature compound (VWR, Suwanee, GA), and flash-frozen in liquid nitrogen. Cryosections from mouse globes or CLNs were cut with a cryostat (HM 500; Micron, Waldorf, Germany), and stored at −80 °C before use. Cultured CD4+ T cells were fixed in acetone for staining. For whole-mount IF staining, the treated corneal and conjunctival tissues were fixed in 10% formaldehyde for 5 min at room temperature (RT) and in acetone for 3 min at −20 °C. IHC or IF staining was performed as previously described respectively.11,50 The primary antibodies used for this study included: rat anti-mouse CD4 from BD Pharmingen (San Jose, CA); rabbit anti-mouse IL-9 from Aviva Systems Biology (San Diego, CA); rabbit anti-mouse IL-9R, IL-33, ZO-1, claudin 1, and occludin from Thermo Fisher Scientific (Waltham, MA); rabbit anti-mouse PU.1, NFATc1, or NFATc2 from Proteintech Group (Rosemont, IL); and rabbit anti-mouse ST2, E-cadherin from Novus Biologicals (Centennial CO). The quantitative analysis for barrier structure was performed by measuring the IF length of each apical junction protein on the images using ImageJ software (Rasband, W.S., ImageJ, US National Institutes of Health, Bethesda, MD, USA, https://imagej.nih.gov/ij/, 1997–2018).
Statistical analysis
Student’s t test was used to compare differences between two groups. One-way ANOVA test was used to make comparisons among three or more groups, followed by Dunnett’s post-hoc test. P values < 0.05 were considered statistically significant.
References
Stern, M. E., Siemasko, K. F. & Niederkorn, J. Y. The Th1/Th2 paradigm in ocular allergy. Curr. Opin. Allergy Clin. Immunol. 5, 446–450 (2005).
Niederkorn, J. Y. Immune regulatory mechanisms in allergic conjunctivitis: insights from mouse models. Curr. Opin. Allergy Clin. Immunol. 8, 472–476 (2008).
Georas, S. N. & Rezaee, F. Epithelial barrier function: at the front line of asthma immunology and allergic airway inflammation. J. Allergy Clin. Immunol. 134, 509–520 (2014).
Takahama, Y., Kosugi, A. & Singer, A. Phenotype, ontogeny, and repertoire of CD4-CD8- T cell receptor alpha beta + thymocytes. Variable influence of self-antigens on T cell receptor V beta usage. J. Immunol. 146, 1134–1141 (1991).
Schmitz, J. et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 23, 479–490 (2005).
Soumelis, V. et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat. Immunol. 3, 673–680 (2002).
Wesemann, D. R. & Nagler, C. R. The microbiome, timing, and barrier function in the context of allergic disease. Immunity 44, 728–738 (2016).
Tan, H. T. et al. Tight junction, mucin, and inflammasome-related molecules are differentially expressed in eosinophilic, mixed, and neutrophilic experimental asthma in mice. Allergy 74, 294–307 (2019).
Zhu, T. H., Zhu, T. R., Tran, K. A., Sivamani, R. K. & Shi, V. Y. Epithelial barrier dysfunctions in atopic dermatitis: a skin-gut-lung model linking microbiome alteration and immune dysregulation. Br. J. Dermatol. 179, 570–581 (2018).
Hufford, M. M. & Kaplan, M. H. A gut reaction to IL-9. Nat. Immunol. 15, 599–600 (2014).
Hu, J. et al. Serine protease inhibitor A3K protects rabbit corneal endothelium from barrier function disruption induced by TNF-alpha. Investig. Ophthalmol. Vis. Sci. 54, 5400–5407 (2013).
Sweerus, K. et al. Claudin-18 deficiency is associated with airway epithelial barrier dysfunction and asthma. J. Allergy Clin. Immunol. 139, 72–81 e1 (2017).
Gerlach, K. et al. TH9 cells that express the transcription factor PU.1 drive T cell-mediated colitis via IL-9 receptor signaling in intestinal epithelial cells. Nat. Immunol. 15, 676–686 (2014).
Wise, S. K. et al. Interleukin-4 and interleukin-13 compromise the sinonasal epithelial barrier and perturb intercellular junction protein expression. Int. Forum Allergy Rhinol. 4, 361–370 (2014).
Dardalhon, V. et al. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(-) effector T cells. Nat. Immunol. 9, 1347–1355 (2008).
Shimbara, A. et al. IL-9 and its receptor in allergic and nonallergic lung disease: increased expression in asthma. J. Allergy Clin. Immunol. 105, 108–115 (2000).
Erpenbeck, V. J. et al. Increased expression of interleukin-9 messenger RNA after segmental allergen challenge in allergic asthmatics. Chest 123, 370S (2003).
Vyas, S. P. & Goswami, R. A decade of Th9 cells: role of Th9 cells in inflammatory bowel disease. Front Immunol. 9, 1139 (2018).
Ciccia, F. et al. Interleukin-9 and T helper type 9 cells in rheumatic diseases. Clin. Exp. Immunol. 185, 125–132 (2016).
Peng, Z., Jiang, S., Wu, M., Zhou, X. & Wang, Q. Expression and role of interleukin-9 in Vogt-Koyanagi-Harada disease. Mol. Vis. 23, 538–547 (2017).
Kaplan, M. H., Hufford, M. M. & Olson, M. R. The development and in vivo function of T helper 9 cells. Nat. Rev. Immunol. 15, 295–307 (2015).
Li, J. et al. IL-9 and Th9 cells in health and diseases-From tolerance to immunopathology. Cytokine Growth Factor Rev. 37, 47–55 (2017).
Koch, S., Sopel, N. & Finotto, S. Th9 and other IL-9-producing cells in allergic asthma. Semin. Immunopathol. 39, 55–68 (2017).
Chang, H. C. et al. PU.1 expression delineates heterogeneity in primary Th2 cells. Immunity 22, 693–703 (2005).
Koch, S., Reppert, S. & Finotto, S. NFATc1 deletion in T lymphocytes inhibits the allergic trait in a murine model of asthma. Clin. Exp. Allergy 45, 1356–1366 (2015).
Karwot, R. et al. Protective role of nuclear factor of activated T cells 2 in CD8+ long-lived memory T cells in an allergy model. J. Allergy Clin. Immunol. 121, 992–9 e6 (2008).
Li, J. et al. Pollen/TLR4 innate immunity signaling initiates IL-33/ST2/Th2 pathways in allergic inflammation. Sci. Rep. 6, 36150 (2016).
Lohning, M. et al. T1/ST2 is preferentially expressed on murine Th2 cells, independent of interleukin 4, interleukin 5, and interleukin 10, and important for Th2 effector function. Proc. Natl Acad. Sci. USA 95, 6930–6935 (1998).
Xu, D. et al. Selective expression of a stable cell surface molecule on type 2 but not type 1 helper T cells. J. Exp. Med. 187, 787–794 (1998).
Turnquist, H. R. et al. IL-1beta-driven ST2L expression promotes maturation resistance in rapamycin-conditioned dendritic cells. J. Immunol. 181, 62–72 (2008).
Rank, M. A. et al. IL-33-activated dendritic cells induce an atypical TH2-type response. J. Allergy Clin. Immunol. 123, 1047–1054 (2009).
Wang, Y. H. Developing food allergy: a potential immunologic pathway linking skin barrier to gut. F1000Res. 5, F1000 (2016).
Loxham, M. & Davies, D. E. Phenotypic and genetic aspects of epithelial barrier function in asthmatic patients. J. Allergy Clin. Immunol. 139, 1736–1751 (2017).
Gerlach, K., McKenzie, A. N., Neurath, M. F. & Weigmann, B. IL-9 regulates intestinal barrier function in experimental T cell-mediated colitis. Tissue Barriers 3, e983777 (2015).
Noelle, R. J. & Nowak, E. C. Cellular sources and immune functions of interleukin-9. Nat. Rev. Immunol. 10, 683–687 (2010).
Blom, L., Poulsen, B. C., Jensen, B. M., Hansen, A. & Poulsen, L. K. IL-33 induces IL-9 production in human CD4+ T cells and basophils. PLoS ONE 6, e21695 (2011).
de Paiva, C. S. et al. Apical corneal barrier disruption in experimental murine dry eye is abrogated by methylprednisolone and doxycycline. Investig. Ophthalmol. Vis. Sci. 47, 2847–2856 (2006).
Reyes, N. J. et al. Neutrophils cause obstruction of eyelid sebaceous glands in inflammatory eye disease in mice. Sci. Transl. Med. 10, eaas9164 (2018).
Doshi, A. et al. Interleukin 9 Alters Epithelial Barrier and E-cadherin in Eosinophilic Esophagitis. J. Pediatr. Gastroenterol. Nutr. 68, 225–231 (2019).
Vermeer, P. D., Harson, R., Einwalter, L. A., Moninger, T. & Zabner, J. Interleukin-9 induces goblet cell hyperplasia during repair of human airway epithelia. Am. J. Respir. Cell Mol. Biol. 28, 286–295 (2003).
Magone, M. T., Chan, C. C., Rizzo, L. V., Kozhich, A. T. & Whitcup, S. M. A novel murine model of allergic conjunctivitis. Clin. Immunol. Immunopathol. 87, 75–84 (1998).
Stern, M. E. et al. Role of interferon-gamma in a mouse model of allergic conjunctivitis. Investig. Ophthalmol. Vis. Sci. 46, 3239–3246 (2005).
Li, D. Q. et al. Short ragweed pollen triggers allergic inflammation through Toll-like receptor 4-dependent thymic stromal lymphopoietin/OX40 ligand/OX40 signaling pathways. J. Allergy Clin. Immunol. 128, 1318–25. e2 (2011).
Zheng, X. et al. TSLP and downstream molecules in experimental mouse allergic conjunctivitis. Investig. Ophthalmol. Vis. Sci. 51, 3076–3082 (2010).
Solomon, A. et al. Doxycycline inhibition of interleukin-1 in the corneal epithelium. Am. J. Ophthalmol. 130, 688 (2000).
Zheng, X., de Paiva, C. S., Li, D. Q., Farley, W. J. & Pflugfelder, S. C. Desiccating stress promotion of Th17 differentiation by ocular surface tissues through a dendritic cell-mediated pathway. Investig. Ophthalmol. Vis. Sci. 51, 3083–3091 (2010).
Krauss, A. H. et al. Improvement of outcome measures of dry eye by a novel integrin antagonist in the Murine Desiccating Stress Model. Investig. Ophthalmol. Vis. Sci. 56, 5888–5895 (2015).
Yoon, K. C. et al. Expression of Th-1 chemokines and chemokine receptors on the ocular surface of C57BL/6 mice: effects of desiccating stress. Investig. Ophthalmol. Vis. Sci. 48, 2561–2569 (2007).
Ma, P. et al. Human corneal epithelium-derived thymic stromal lymphopoietin links the innate and adaptive immune responses via TLRs and Th2 cytokines. Investig. Ophthalmol. Vis. Sci. 50, 2702–2709 (2009).
de Paiva, C. S., Chen, Z., Corrales, R. M., Pflugfelder, S. C. & Li, D. Q. ABCG2 transporter identifies a population of clonogenic human limbal epithelial cells. Stem Cells 23, 63–73 (2005).
Acknowledgements
We would like to thank Dr. Andrew N.J. McKenzie, FMedSci at Division of Protein and Nucleic Acid Chemistry, MRC Laboratory of Molecular Biology, Cambridge, UK, for kindly providing the homozygote ST2−/− mice in BALB/c background through Dr Stephanie Cormier at the University of Tennessee, USA. This work was partially supported by National Institutes of Health, National Eye Institute grants R01 EY023598 (DQL), and Core Grant for Vision Research EY002520, an unrestricted grant from Research to Prevent Blindness, the Oshman Foundation and the William Stamps Farish Fund.
Author information
Authors and Affiliations
Contributions
D.-Q.L. and S.C.P designed the research; J.H., N.G., Y.Z., X.C., J.L, F.B. and W.C. performed the experiments; J.H., N.G., Y.Z., J.L, Z.L, C.D.P. and D.-Q.L. analyzed the data and prepared figures; J.H. and D.-Q.L. wrote the paper; S.C.P. performed English editing; and all authors reviewed the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hu, J., Gao, N., Zhang, Y. et al. IL-33/ST2/IL-9/IL-9R signaling disrupts ocular surface barrier in allergic inflammation. Mucosal Immunol 13, 919–930 (2020). https://doi.org/10.1038/s41385-020-0288-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41385-020-0288-4
This article is cited by
-
An ocular Th1 immune response promotes corneal nerve damage independently of the development of corneal epitheliopathy
Journal of Neuroinflammation (2023)
-
Mucosal immunology of the ocular surface
Mucosal Immunology (2022)
-
Development of an ultra-sensitive human IL-33 biomarker assay for age-related macular degeneration and asthma drug development
Journal of Translational Medicine (2021)