Abstract
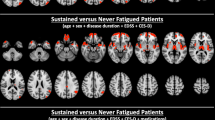
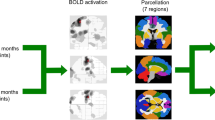
Dysregulation of monoaminergic networks might have a role in the pathogenesis of fatigue in multiple sclerosis (MS). We investigated longitudinal changes of resting state (RS) functional connectivity (FC) in monoaminergic networks and their association with the development of fatigue in MS. Eighty-nine MS patients and 49 age- and sex-matched healthy controls (HC) underwent neurological, fatigue, and RS functional MRI assessment at baseline and after a median follow-up of 1.3 years (interquartile range = 1.01–2.01 years). Monoaminergic-related RS FC was estimated with an independent component analysis constrained to PET atlases for dopamine (DA), noradrenaline (NA), and serotonin (5-HT) transporters. At baseline, 24 (27%) MS patients were fatigued (F) and 65 were not fatigued (NF). Of these, 22 (34%) developed fatigue (DEV-FAT) at follow-up and 43 remained not fatigued (NO-FAT). At baseline, F-MS patients showed increased monoaminergic-related RS FC in the caudate nucleus vs NF-MS and in the hippocampal, postcentral, temporal, and occipital cortices vs NF-MS and HC. Moreover, F-MS patients exhibited decreased RS FC in the frontal cortex vs NF-MS and HC, and in the thalamus vs NF-MS. During the follow-up, no RS FC changes were observed in HC. NO-FAT patients showed limited DA-related RS FC modifications, whereas DEV-FAT MS patients showed increased DA-related RS FC in the left hippocampus, significant at time-by-group interaction analysis. In the NA-related network, NO-FAT patients showed decreased RS FC over time in the left superior frontal gyrus. This region showed increased RS FC in both DEV-FAT and F-MS patients; this divergent behavior was significant at time-by-group interaction analysis. Finally, DEV-FAT MS patients presented increased 5-HT-related RS FC in the angular and middle occipital gyri, while this latter region showed decreased 5-HT-related RS FC during the follow-up in F-MS patients. In MS patients, distinct patterns of alterations were observed in monoaminergic networks based on their fatigue status. Fatigue was closely linked to specific changes in the basal ganglia and hippocampal, superior frontal, and middle occipital cortices.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The anonymized dataset used and analyzed during the current study is available from the corresponding author upon reasonable request.
References
Oliva Ramirez A, Keenan A, Kalau O, Worthington E, Cohen L, Singh S. Prevalence and burden of multiple sclerosis-related fatigue: a systematic literature review. BMC Neurol. 2021;21:468.
Marchesi O, Vizzino C, Meani A, Conti L, Riccitelli GC, Preziosa P, et al. Fatigue in multiple sclerosis patients with different clinical phenotypes: a clinical and magnetic resonance imaging study. Eur J Neurol. 2020;27:2549–60.
Roelcke U, Kappos L, Lechner-Scott J, Brunnschweiler H, Huber S, Ammann W, et al. Reduced glucose metabolism in the frontal cortex and basal ganglia of multiple sclerosis patients with fatigue: a 18F-fluorodeoxyglucose positron emission tomography study. Neurology. 1997;48:1566–71.
Tellez N, Alonso J, Rio J, Tintore M, Nos C, Montalban X, et al. The basal ganglia: a substrate for fatigue in multiple sclerosis. Neuroradiology. 2008;50:17–23.
Tartaglia MC, Narayanan S, Francis SJ, Santos AC, De Stefano N, Lapierre Y, et al. The relationship between diffuse axonal damage and fatigue in multiple sclerosis. Arch Neurol. 2004;61:201–7.
Finke C, Schlichting J, Papazoglou S, Scheel M, Freing A, Soemmer C, et al. Altered basal ganglia functional connectivity in multiple sclerosis patients with fatigue. Mult Scler. 2015;21:925–34.
Rocca MA, Meani A, Riccitelli GC, Colombo B, Rodegher M, Falini A, et al. Abnormal adaptation over time of motor network recruitment in multiple sclerosis patients with fatigue. Mult Scler. 2016;22:1144–53.
Cruz Gomez AJ, Ventura Campos N, Belenguer A, Avila C, Forn C. Regional brain atrophy and functional connectivity changes related to fatigue in multiple sclerosis. PLoS ONE. 2013;8:e77914.
Pravata E, Zecca C, Sestieri C, Caulo M, Riccitelli GC, Rocca MA, et al. Hyperconnectivity of the dorsolateral prefrontal cortex following mental effort in multiple sclerosis patients with cognitive fatigue. Mult Scler. 2016;22:1665–75.
Engstrom M, Flensner G, Landtblom AM, Ek AC, Karlsson T. Thalamo-striato-cortical determinants to fatigue in multiple sclerosis. Brain Behav. 2013;3:715–28.
Arm J, Ribbons K, Lechner-Scott J, Ramadan S. Evaluation of MS related central fatigue using MR neuroimaging methods: scoping review. J Neurol Sci. 2019;400:52–71.
Bertoli M, Tecchio F. Fatigue in multiple sclerosis: does the functional or structural damage prevail? Mult Scler. 2020;26:1809–15.
Filippi M, Preziosa P, Rocca MA. Brain mapping in multiple sclerosis: lessons learned about the human brain. Neuroimage. 2019;190:32–45.
White AT, Lee JN, Light AR, Light KC. Brain activation in multiple sclerosis: a BOLD fMRI study of the effects of fatiguing hand exercise. Mult Scler. 2009;15:580–6.
Filippi M, Rocca MA, Colombo B, Falini A, Codella M, Scotti G, et al. Functional magnetic resonance imaging correlates of fatigue in multiple sclerosis. Neuroimage. 2002;15:559–67.
Hidalgo de la Cruz M, d’Ambrosio A, Valsasina P, Pagani E, Colombo B, Rodegher M, et al. Abnormal functional connectivity of thalamic sub-regions contributes to fatigue in multiple sclerosis. Mult Scler. 2018;24:1183–95.
DeLuca J, Genova HM, Hillary FG, Wylie G. Neural correlates of cognitive fatigue in multiple sclerosis using functional MRI. J Neurol Sci. 2008;270:28–39.
Genova HM, Rajagopalan V, Deluca J, Das A, Binder A, Arjunan A, et al. Examination of cognitive fatigue in multiple sclerosis using functional magnetic resonance imaging and diffusion tensor imaging. PLoS ONE. 2013;8:e78811.
Arm J, Oeltzschner G, Al-Iedani O, Lea R, Lechner-Scott J, Ramadan S. Altered in vivo brain GABA and glutamate levels are associated with multiple sclerosis central fatigue. Eur J Radiol. 2021;137:109610.
Carotenuto A, Valsasina P, Preziosa P, Mistri D, Filippi M, Rocca MA. Monoaminergic network abnormalities: a marker for multiple sclerosis-related fatigue and depression. J Neurol Neurosurg Psychiatry. 2023;94:94–101.
Cercignani M, Dipasquale O, Bogdan I, Carandini T, Scott J, Rashid W, et al. Cognitive fatigue in multiple sclerosis is associated with alterations in the functional connectivity of monoamine circuits. Brain Commun. 2021;3:fcab023.
Fiore A, Preziosa P, Tedone N, Margoni M, Vizzino C, Mistri D, et al. Correspondence among gray matter atrophy and atlas-based neurotransmitter maps is clinically relevant in multiple sclerosis. Mol Psychiatry. 2023;28:1770–82.
Flachenecker P, Kumpfel T, Kallmann B, Gottschalk M, Grauer O, Rieckmann P, et al. Fatigue in multiple sclerosis: a comparison of different rating scales and correlation to clinical parameters. Mult Scler. 2002;8:523–6.
Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2:125–41.
Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37:90–101.
Gómez FJG, Huertas I, Ramírez JAL, Solís DG. Elaboración de una plantilla de SPM para la normalización de imágenes de PET con 18F-DOPA. Imagen Diagnóstica. 2018;9:23–25.
Hesse S, Moeller F, Petroff D, Lobsien D, Luthardt J, Regenthal R, et al. Altered serotonin transporter availability in patients with multiple sclerosis. Eur J Nucl Med Mol Imaging. 2014;41:827–35.
Schmidt E, Schinke C, Rullmann M, Luthardt J, Becker GA, Haars S, et al. Changes of central noradrenaline transporter availability in immunotherapy-naive multiple sclerosis patients. Sci Rep. 2020;10:14651.
Carandini T, Mancini M, Bogdan I, Rae CL, Barritt AW, Sethi A, et al. Disruption of brainstem monoaminergic fibre tracts in multiple sclerosis as a putative mechanism for cognitive fatigue: a fixel-based analysis. Neuroimage Clin. 2021;30:102587.
Jaeger S, Paul F, Scheel M, Brandt A, Heine J, Pach D, et al. Multiple sclerosis-related fatigue: altered resting-state functional connectivity of the ventral striatum and dorsolateral prefrontal cortex. Mult Scler. 2019;25:554–64.
Rocca MA, Valsasina P, Lamanna MT, Colombo B, Martinelli V, Filippi M. Functional connectivity modifications in monoaminergic circuits occur in fatigued MS patients treated with fampridine and amantadine. J Neurol. 2023;270:4697–706.
Stefancin P, Govindarajan ST, Krupp L, Charvet L, Duong TQ. Resting-state functional connectivity networks associated with fatigue in multiple sclerosis with early age onset. Mult Scler Relat Disord. 2019;31:101–5.
Rocca MA, Valsasina P, Colombo B, Martinelli V, Filippi M. Cortico-subcortical functional connectivity modifications in fatigued multiple sclerosis patients treated with fampridine and amantadine. Eur J Neurol. 2021;28:2249–58.
Minagar A, Barnett MH, Benedict RH, Pelletier D, Pirko I, Sahraian MA, et al. The thalamus and multiple sclerosis: modern views on pathologic, imaging, and clinical aspects. Neurology. 2013;80:210–9.
Zhou K, Zhu L, Hou G, Chen X, Chen B, Yang C, et al. The contribution of thalamic nuclei in salience processing. Front Behav Neurosci. 2021;15:634618.
Rocca MA, Agosta F, Colombo B, Mezzapesa DM, Falini A, Comi G, et al. fMRI changes in relapsing-remitting multiple sclerosis patients complaining of fatigue after IFNbeta-1a injection. Hum Brain Mapp. 2007;28:373–82.
Schoonheim MM, Meijer KA, Geurts JJ. Network collapse and cognitive impairment in multiple sclerosis. Front Neurol. 2015;6:82.
Chahin S, Miller D, Sakai RE, Wilson JA, Frohman T, Markowitz C, et al. Relation of quantitative visual and neurologic outcomes to fatigue in multiple sclerosis. Mult Scler Relat Disord. 2015;4:304–10.
Funding
The study described in the present manuscript received no funding.
Author information
Authors and Affiliations
Contributions
MM, PV, and AB contributed to the acquisition, analysis, and interpretation of MRI data, drafting and revising the text, and preparing the figures. DM and PP contributed to the acquisition, analysis, and interpretation of clinical and MRI data and revising the text. MAR and MF contributed to the conception of the study, drafting and revising the text, acting as the study supervisors. All the authors gave their approval to the current version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
MM reports grants and personal fees from Sanofi Genzyme, Merck Serono, Novartis, and Almiral. She was awarded a MAGNIMS-ECTRIMS fellowship in 2020. PV received speakers’ honoraria from Biogen Idec. AB and DM have nothing to disclose. PP received speaker honoraria from Roche, Biogen, Novartis, Merck Serono, Bristol Myers Squibb, Genzyme, Horizon, and Sanofi. He has received research support from Italian Ministry of Health and Fondazione Italiana Sclerosi Multipla. MAR received consulting fees from Biogen, Bristol Myers Squibb, Eli Lilly, Janssen, Roche; and speaker honoraria from Almirall, Bayer, Biogen, Bristol Myers Squibb, Bromatech, Celgene, Genzyme, Merck Healthcare Germany, Merck Serono SpA, Novartis, Roche, and Teva. She receives research support from the MS Society of Canada, the Italian Ministry of Health, The Italian Ministry of University and Research, and Fondazione Italiana Sclerosi Multipla. She is Associate Editor for Multiple Sclerosis and Related Disorders. MF is Editor-in-Chief of the Journal of Neurology, Associate Editor of Human Brain Mapping, Associate Editor of Radiology, and Associate Editor of Neurological Sciences; received compensation for consulting services from Alexion, Almirall, Biogen, Merck, Novartis, Roche, Sanofi; speaking activities from Bayer, Biogen, Celgene, Chiesi Italia SpA, Eli Lilly, Genzyme, Janssen, Merck Serono, Neopharmed Gentili, Novartis, Novo Nordisk, Roche, Sanofi, Takeda, and TEVA; participation in Advisory Boards for Alexion, Biogen, Bristol Myers Squibb, Merck, Novartis, Roche, Sanofi, Sanofi-Aventis, Sanofi Genzyme, Takeda; scientific direction of educational events for Biogen, Merck, Roche, Celgene, Bristol Myers Squibb, Lilly, Novartis, Sanofi Genzyme; he receives research support from Biogen Idec, Merck Serono, Novartis, Roche, the Italian Ministry of Health, the Italian Ministry of University and Research, and Fondazione Italiana Sclerosi Multipla.
Ethical approval
Approval was received from the institutional ethical standards committee on human experimentation of IRCCS Ospedale San Raffaele for any experiments using human subjects (Protocol N° 2015-33). Written informed consent was obtained from all subjects prior to study participation according to the Declaration of Helsinki.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Margoni, M., Valsasina, P., Bacchetti, A. et al. Resting state functional connectivity modifications in monoaminergic circuits underpin fatigue development in patients with multiple sclerosis. Mol Psychiatry (2024). https://doi.org/10.1038/s41380-024-02532-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41380-024-02532-6