Abstract
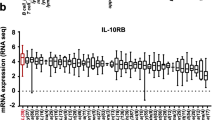
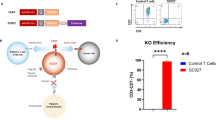
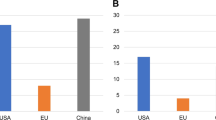
C-type lectin-like molecule-1 (CLL1) is preferentially expressed on acute myeloid leukemia (AML) stem cells and AML blasts, which can be considered as AML-associated antigen. Anti-CLL1-based CAR-T cells exhibited effective tumor-killing capacity in vitro and in AML-bearing mouse model. In this report, eight children with relapsed or refractory AML (R/R-AML) were recruited for a phase 1/2 clinical trial of autologous anti-CLL1 CAR-T cell immunotherapy. The objectives of this clinical trial were to evaluate the safety and the preliminary efficacy of anti-CLL1 CAR-T cell treatment. Patients received one dose of autologous anti-CLL1 CAR-T cells after lymphodepletion conditioning. After CAR-T treatment, patients developed grade 1–2 cytokine release syndrome (CRS) but without any lethal events. 4 out of 8 patients achieved morphologic leukemia-free state (MLFS) and minimal residual disease (MRD) negativity, 1 patient with MLFS and MRD positivity, 1 patient achieved complete remission with incomplete hematologic recovery (CRi) but MRD positivity, 1 patient with partial remission (PR), and 1 patient remained at stable disease (SD) status but had CLL1-positive AML blast clearance. These results suggested that anti-CLL1-based CAR-T cell immunotherapy can be considered as a well-tolerated and effective option for treating children with R/R-AML.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files. Further inquiries can be directed to the corresponding authors.
References
Bonaventure A, Harewood R, Stiller CA, Gatta G, Clavel J, Stefan DC, et al. Worldwide comparison of survival from childhood leukaemia for 1995-2009, by subtype, age, and sex (CONCORD-2): a population-based study of individual data for 89 828 children from 198 registries in 53 countries. Lancet Haematol. 2017;4:e202–e17.
Rubnitz JE, Inaba H, Dahl G, Ribeiro RC, Bowman WP, Taub J, et al. Minimal residual disease-directed therapy for childhood acute myeloid leukaemia: results of the AML02 multicentre trial. Lancet Oncol. 2010;11:543–52.
Rasche M, Zimmermann M, Borschel L, Bourquin JP, Dworzak M, Klingebiel T, et al. Successes and challenges in the treatment of pediatric acute myeloid leukemia: a retrospective analysis of the AML-BFM trials from 1987 to 2012. Leukemia 2018;32:2167–77.
Creutzig U, Zimmermann M, Dworzak MN, Gibson B, Tamminga R, Abrahamsson J, et al. The prognostic significance of early treatment response in pediatric relapsed acute myeloid leukemia: results of the international study Relapsed AML 2001/01. Haematologica 2014;99:1472–8.
Short NJ, Rytting ME, Cortes JE. Acute myeloid leukaemia. Lancet 2018;392:593–606.
Moors I, Vandepoele K, Philippe J, Deeren D, Selleslag D, Breems D, et al. Clinical implications of measurable residual disease in AML: Review of current evidence. Crit Rev Oncol Hematol. 2019;133:142–8.
Short NJ, Konopleva M, Kadia TM, Borthakur G, Ravandi F, DiNardo CD, et al. Advances in the treatment of acute myeloid leukemia: new drugs and new challenges. Cancer Discov. 2020;10:506–25.
Karol SE, Alexander TB, Budhraja A, Pounds SB, Canavera K, Wang L, et al. Venetoclax in combination with cytarabine with or without idarubicin in children with relapsed or refractory acute myeloid leukaemia: a phase 1, dose-escalation study. Lancet Oncol 2020;21:551–60.
Niktoreh N, Lerius B, Zimmermann M, Gruhn B, Escherich G, Bourquin JP, et al. Gemtuzumab ozogamicin in children with relapsed or refractory acute myeloid leukemia: a report by Berlin-Frankfurt-Munster study group. Haematologica 2019;104:120–7.
van Eijkelenburg NKA, Rasche M, Ghazaly E, Dworzak MN, Klingebiel T, Rossig C, et al. Clofarabine, high-dose cytarabine and liposomal daunorubicin in pediatric relapsed/refractory acute myeloid leukemia: a phase IB study. Haematologica 2018;103:1484–92.
Raje N, Berdeja J, Lin Y, Siegel D, Jagannath S, Madduri D, et al. Anti-BCMA CAR T-Cell Therapy bb2121 in relapsed or refractory multiple myeloma. N. Engl J Med. 2019;380:1726–37.
Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-Cell Lymphoma. N. Engl J Med. 2019;380:45–56.
Ying Z, Huang XF, Xiang X, Liu Y, Kang X, Song Y, et al. A safe and potent anti-CD19 CAR T cell therapy. Nat Med 2019;25:947–53.
Wang Z, Wu Z, Liu Y, Han W. New development in CAR-T cell therapy. J Hematol Oncol. 2017;10:53.
Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in children and young adults with B-Cell lymphoblastic leukemia. N. Engl J Med. 2018;378:439–48.
Park JH, Riviere I, Gonen M, Wang X, Senechal B, Curran KJ, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N. Engl J Med. 2018;378:449–59.
Shah NN, Fry TJ. Mechanisms of resistance to CAR T cell therapy. Nat Rev Clin Oncol. 2019;16:372–85.
van Rhenen A, van Dongen GA, Kelder A, Rombouts EJ, Feller N, Moshaver B, et al. The novel AML stem cell associated antigen CLL-1 aids in discrimination between normal and leukemic stem cells. Blood 2007;110:2659–66.
Leong SR, Sukumaran S, Hristopoulos M, Totpal K, Stainton S, Lu E, et al. An anti-CD3/anti-CLL-1 bispecific antibody for the treatment of acute myeloid leukemia. Blood 2017;129:609–18.
Jiang YP, Liu BY, Zheng Q, Panuganti S, Chen R, Zhu J, et al. CLT030, a leukemic stem cell-targeting CLL1 antibody-drug conjugate for treatment of acute myeloid leukemia. Blood Adv. 2018;2:1738–49.
Lin TY, Zhu Y, Li Y, Zhang H, Ma AH, Long Q, et al. Daunorubicin-containing CLL1-targeting nanomicelles have anti-leukemia stem cell activity in acute myeloid leukemia. Nanomedicine 2019;20:102004.
Tashiro H, Sauer T, Shum T, Parikh K, Mamonkin M, Omer B, et al. Treatment of acute myeloid leukemia with T cells expressing chimeric antigen receptors directed to C-type Lectin-like Molecule 1. Mol Ther. 2017;25:2202–13.
Wang J, Chen S, Xiao W, Li W, Wang L, Yang S, et al. CAR-T cells targeting CLL-1 as an approach to treat acute myeloid leukemia. J Hematol Oncol. 2018;11:7.
Zhang H, Wang P, Li Z, He Y, Gan W, Jiang H. Anti-CLL1 chimeric antigen receptor T-Cell therapy in children with relapsed/refractory acute myeloid leukemia. Clin Cancer Res. 2021;27:3549–55.
Liu F, Cao Y, Pinz KG, Ma Y, Wada M, Chen KH, et al. First-in-Human CLL1-CD33 Compound CAR T cell therapy induces complete remission in patients with refractory acute myeloid leukemia: update on Phase 1 clinical trial. Am Soc Hematol Annu Meet: Blood. 2018;132:901.
Zhang H, Gan WT, Hao WG, Wang PF, Li ZY, Chang LJ. Successful Anti-CLL1 CAR T-cell therapy in secondary acute myeloid leukemia. Front Oncol. 2020;10:685.
Dohner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Buchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017;129:424–47.
Mahadeo KM, Khazal SJ, Abdel-Azim H, Fitzgerald JC, Taraseviciute A, Bollard CM, et al. Management guidelines for paediatric patients receiving chimeric antigen receptor T cell therapy. Nat Rev Clin Oncol. 2019;16:45–63.
Neelapu SS, Tummala S, Kebriaei P, Wierda W, Gutierrez C, Locke FL, et al. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15:47–62.
Hofmann S, Schubert ML, Wang L, He B, Neuber B, Dreger P, et al. Chimeric Antigen Receptor (CAR) T Cell Therapy in Acute Myeloid Leukemia (AML). J Clin Med. 2019;8:200.
Mardiana S, Gill S. CAR T cells for acute myeloid leukemia: state of the art and future directions. Front Oncol. 2020;10:697.
Sallman DA, Brayer J, Sagatys EM, Lonez C, Breman E, Agaugue S, et al. NKG2D-based chimeric antigen receptor therapy induced remission in a relapsed/refractory acute myeloid leukemia patient. Haematologica 2018;103:e424–e6.
Budde L, Song JY, Kim Y, Blanchard S, Wagner J, Stein AS, et al. Remissions of acute myeloid leukemia and blastic plasmacytoid dendritic cell neoplasm following treatment with CD123-specific CAR T cells: a first-in-human clinical trial. Am Soc Hematol Annu Meet: Blood. 2017;130:811.
Liu F, Zhang H, Sun L, Li Y, Zhang S, He G, et al. First-in-human CLL1-CD33 compound CAR (cCAR) T cell therapy in relapsed and refractory acute myeloid leukemia. European Hematology Association Meeting; 06/12/202020. S149.
Rubin DB, Danish HH, Ali AB, Li K, LaRose S, Monk AD, et al. Neurological toxicities associated with chimeric antigen receptor T-cell therapy. Brain 2019;142:1334–48.
Belin C, Devic P, Ayrignac X, Dos Santos A, Paix A, Sirven-Villaros L, et al. Description of neurotoxicity in a series of patients treated with CAR T-cell therapy. Sci Rep. 2020;10:18997.
Petrov JC, Wada M, Pinz KG, Yan LE, Chen KH, Shuai X, et al. Compound CAR T-cells as a double-pronged approach for treating acute myeloid leukemia. Leukemia 2018;32:1317–26.
Ma H, Padmanabhan IS, Parmar S, Gong Y. Targeting CLL-1 for acute myeloid leukemia therapy. J Hematol Oncol. 2019;12:41.
Wang J, Hu Y, Huang H. Acute lymphoblastic leukemia relapse after CD19-targeted chimeric antigen receptor T cell therapy. J Leukoc Biol. 2017;102:1347–56.
Zhang LN, Song Y, Liu D. CD19 CAR-T cell therapy for relapsed/refractory acute lymphoblastic leukemia: factors affecting toxicities and long-term efficacies. J Hematol Oncol. 2018;11:41.
Pan J, Yang JF, Deng BP, Zhao XJ, Zhang X, Lin YH, et al. High efficacy and safety of low-dose CD19-directed CAR-T cell therapy in 51 refractory or relapsed B acute lymphoblastic leukemia patients. Leukemia 2017;31:2587–93.
Hay KA, Gauthier J, Hirayama AV, Voutsinas JM, Wu Q, Li D, et al. Factors associated with durable EFS in adult B-cell ALL patients achieving MRD-negative CR after CD19 CAR T-cell therapy. Blood 2019;133:1652–63.
Ma Y, Zhang S, Fang H, Yu K, Jiang S. A phase I study of CAR-T bridging HSCT in patients with acute CD19(+) relapse/refractory B-cell leukemia. Oncol Lett. 2020;20:20.
Jin X, Zhang M, Sun R, Lyu H, Xiao X, Zhang X, et al. First-in-human phase I study of CLL-1 CAR-T cells in adults with relapsed/refractory acute myeloid leukemia. J Hematol Oncol. 2022;15:88.
Acknowledgements
We would like to thank all the patients and their parents for their participation. This work was sponsored by Natural Science Foundation of Xinjiang (NO.2021D01C351), and supported by Guangzhou Science & Technology Project (202206010141), and partially funded by research funds from St. Baldrick’s Foundation International Scholar (581580), Guangzhou Women and Children’s Medical Center Internal Program (IP-2018-001), and Pearl River S&T Nova Program of Guangzhou (201906010056).
Funding
This work was sponsored by Natural Science Foundation of Xinjiang (NO.2021D01C351), and supported by Guangzhou Science & Technology Project (202206010141), and partially funded by research funds from St. Baldrick’s Foundation International Scholar (581580), Guangzhou Women and Children’s Medical Center Internal Program (IP-2018-001), and Pearl River S&T Nova Program of Guangzhou (201906010056). This work was also partially supported by grant from National Natural Science Foundation of China (82170152).
Author information
Authors and Affiliations
Contributions
The study was conceived by HZ and CL, designed by HZ, CL, and ML, supervised by HZ, CL, and ML. HZ, CB, ZP, GL and CL performed the research. HZ, CB, ZP, YH, ZH, and KP recruited the patients and collected clinical data. Data was conducted and interpreted by HZ, CB, ZP, GL, ZZ, WD, ML and CL. HZ, CL, YZ and ML wrote the manuscript. All authors approved the final version for publication.
Corresponding authors
Ethics declarations
Competing interests
GL, ZZ, WD, YZ and ML are employees of Guangzhou Bio-Gene Technology Co., Ltd., who have potential interest, while other authors have nothing to disclose.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, H., Bu, C., Peng, Z. et al. Characteristics of anti-CLL1 based CAR-T therapy for children with relapsed or refractory acute myeloid leukemia: the multi-center efficacy and safety interim analysis. Leukemia 36, 2596–2604 (2022). https://doi.org/10.1038/s41375-022-01703-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-022-01703-0
This article is cited by
-
Targeted Therapies in Pediatric Acute Myeloid Leukemia - Evolving Therapeutic Landscape
Indian Journal of Pediatrics (2024)
-
Modified EASIX scores predict severe CRS/ICANS in patients with acute myeloid leukemia following CLL1 CAR-T cell therapy
Annals of Hematology (2024)