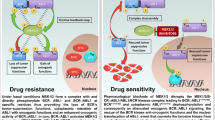
Abstract
Idelalisib targets PI3Kδ in the BCR pathway generating only a partial response in CLL patients, indicating that the leukemic cells may have evolved escape signals. Indeed, we detected increased activation of AKT accompanied by upregulation of MYC/BCL2 in post-therapy CLL cells from patients treated with idelalisib/ofatumumab. To unravel the mechanism of increased AKT-activation, we studied the impact of idelalisib on a CLL-derived cell line, MEC1, as a model. After an initial inhibition, AKT-activation level was restored in idelalisib-treated MEC1 cells in a time-dependent manner. As BCAP (B-cell adaptor for PI3K) and CD19 recruit PI3Kδ to activate AKT upon BCR-stimulation, we examined if idelalisib-treatment altered PI3Kδ-recruitment. Immunoprecipitation of BCAP/CD19 from idelalisib-treated MEC1 cells showed increased recruitment of PI3Kδ in association with PI3Kβ, but not PI3Kα or PI3Kγ and that, targeting both PI3Kδ with PI3Kβ inhibited AKT-reactivation. We detected similar, patient-specific recruitment pattern of PI3K-isoforms by BCAP/CD19 in post-idelalisib CLL cells with increased AKT-activation. Interestingly, a stronger inhibitory effect of idelalisib on P-AKT (T308) than S473 was discernible in idelalisib-treated cells despite increased recruitment of PI3Kδ/PI3Kβ and accumulation of phosphatidylinositol-3,4,5-triphosphate; which could be attributed to reduced PDK1 activity. Thus, administration of isoform-specific inhibitors may prove more effective strategy for treating CLL patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Burger JA, Chiorazzi N. B cell receptor signaling in chronic lymphocytic leukemia. Trends Immunol. 2013;34:592–601.
Maffei R, Fiorcari S, Martinelli S, Potenza L, Luppi M, Marasca R. Targeting neoplastic B cells and harnessing microenvironment: the “double face” of ibrutinib and idelalisib. J Hematol Oncol. 2015;8:1–13.
Ten Hacken E, Gounari M, Ghia P, Burger JA. The importance of B cell receptor isotypes and stereotypes in chronic lymphocytic leukemia. Leukemia 2019;33:287–98.
Lucas CL, Chandra A, Nejentsev S, Condliffe AM, Okkenhaug K. PI3Kdelta and primary immunodeficiencies. Nat Rev Immunol. 2016;16:702–14.
Thorpe LM, Yuzugullu H, Zhao JJ. PI3K in cancer: Divergent roles of isoforms, modes of activation and therapeutic targeting. Nat Rev Cancer. 2015;15:7–24.
Herman SE, Johnson AJ. Molecular pathways: Targeting phosphoinositide 3-kinase p110-delta in chronic lymphocytic leukemia. Clin Cancer Res. 2012;18:4013–8.
Okkenhaug K, Vanhaesebroeck B. PI3K in lymphocyte development, differentiation and activation. Nat Rev Immunol. 2003;3:317–30.
Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–19.
Fruman DA, Chiu H, Hopkins BD, Bagrodia S, Cantley LC, Abraham RT. The PI3K pathway in human disease. Cell 2017;170:605–35.
Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, et al. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–51.
Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, et al. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997;7:261–9.
Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 2005;307:1098–101.
Manning BD, Toker A. AKT/PKB signaling: Navigating the network. Cell 2017;169:381–405.
Lannutti BJ, Meadows SA, Herman SE, Kashishian A, Steiner B, Johnson AJ, et al. CAL-101, a p110delta selective phosphatidylinositol-3-kinase inhibitor for the treatment of B-cell malignancies, inhibits PI3K signaling and cellular viability. Blood 2011;117:591–4.
Herman SE, Gordon AL, Wagner AJ, Heerema NA, Zhao W, Flynn JM, et al. Phosphatidylinositol 3-kinase-delta inhibitor CAL-101 shows promising preclinical activity in chronic lymphocytic leukemia by antagonizing intrinsic and extrinsic cellular survival signals. Blood 2010;116:2078–88.
Brown JR, Byrd JC, Coutre SE, Benson DM, Flinn IW, Wagner-Johnston ND, et al. Idelalisib, an inhibitor of phosphatidylinositol 3 kinase p110delta, for relapsed/refractory chronic lymphocytic leukemia. Blood. 2014;123:3390–7.
Stacchini A, Aragno M, Vallario A, Alfarano A, Circosta P, Gottardi D, et al. MEC1 and MEC2: Two new cell lines derived from B-chronic lymphocytic leukaemia in prolymphocytoid transformation. Leuk Res. 1999;23:127–36.
Mahmud H, Mendez M, Mukhopadhyay B, Holter-Chakrabarty J, Ghosh AK. HSP90 overexpression potentiates the B-cell receptor and fibroblast growth factor receptor survival signals in chronic lymphocytic leukemia cells. Oncotarget 2020;11:2037–46.
Maiti GP, Sinha S, Mahmud H, Boysen J, Mendez MT, Vesely SK, et al. SIRT3 overexpression and epigenetic silencing of catalase regulate ROS accumulation in CLL cells activating AXL signaling axis. Blood. Cancer J. 2021;11:93.
Rickert RC. New insights into pre-BCR and BCR signalling with relevance to B cell malignancies. Nat Rev Immunol. 2013;13:578–91.
Aiba Y, Kameyama M, Yamazaki T, Tedder TF, Kurosaki T. Regulation of B-cell development by BCAP and CD19 through their binding to phosphoinositide 3-kinase. Blood 2008;111:1497–503.
Chu N, Salguero AL, Liu AZ, Chen Z, Dempsey DR, Ficarro SB, et al. Akt kinase activation mechanisms revealed using protein semisynthesis. Cell 2018;174:897–907.e14.
Hoellenriegel J, Meadows SA, Sivina M, Wierda WG, Kantarjian H, Keating MJ, et al. The phosphoinositide 3’-kinase delta inhibitor, CAL-101, inhibits B-cell receptor signaling and chemokine networks in chronic lymphocytic leukemia. Blood 2011;118:3603–12.
Niiro H, Clark EA. Regulation of B-cell fate by antigen-receptor signals. Nat Rev Immunol. 2002;2:945–56.
Dal Porto JM, Gauld SB, Merrell KT, Mills D, Pugh-Bernard AE, Cambier J. B cell antigen receptor signaling 101. Mol Immunol. 2004;41:599–613.
Geering B, Cutillas PR, Vanhaesebroeck B. Regulation of class IA PI3Ks: Is there a role for monomeric PI3K subunits? Biochem Soc Trans. 2007;35:199–203.
Pongas GN, Annunziata CM, Staudt LM. PI3Kdelta inhibition causes feedback activation of PI3Kalpha in the ABC subtype of diffuse large B-cell lymphoma. Oncotarget 2017;8:81794–802.
Dbouk HA, Pang H, Fiser A, Backer JM. A biochemical mechanism for the oncogenic potential of the p110beta catalytic subunit of phosphoinositide 3-kinase. Proc Natl Acad Sci USA 2010;107:19897–902.
Zhang X, Vadas O, Perisic O, Anderson KE, Clark J, Hawkins PT, et al. Structure of lipid kinase p110beta/p85beta elucidates an unusual SH2-domain-mediated inhibitory mechanism. Mol Cell. 2011;41:567–78.
Vogt PK. PI3K p110beta: More tightly controlled or constitutively active? Mol Cell. 2011;41:499–501.
Kang S, Denley A, Vanhaesebroeck B, Vogt PK. Oncogenic transformation induced by the p110beta, -gamma, and -delta isoforms of class I phosphoinositide 3-kinase. Proc Natl Acad Sci USA 2006;103:1289–94.
Denley A, Kang S, Karst U, Vogt PK. Oncogenic signaling of class I PI3K isoforms. Oncogene 2008;27:2561–74.
Visconti L, Malagrino F, Toto A, Gianni S. The kinetics of folding of the NSH2 domain from p85. Sci Rep. 2019;9:4058.
Scheffold A, Jebaraj BMC, Tausch E, Bloehdorn J, Ghia P, Yahiaoui A, et al. IGF1R as druggable target mediating PI3K-delta inhibitor resistance in a murine model of chronic lymphocytic leukemia. Blood 2019;134:534–47.
Ishwarya M, Siddha K, Aishath N, Svitlana T, Jasneet KK, Emily MT, et al. Activating MAPK pathway mutations mediate primary resistance to PI3K inhibitors in Chronic Lymphocytic Leukemia (CLL). Blood. 2018;132(Supplement 1):587.
Edelmann J, Dokal AD, Vilventhraraja E, Holzmann K, Britton D, Klymenko T, et al. Rituximab and obinutuzumab differentially hijack the B cell receptor and NOTCH1 signaling pathways. iScience. 2021;24:102089.
Pavlasova G, Borsky M, Svobodova V, Oppelt J, Cerna K, Novotna J, et al. Rituximab primarily targets an intra-clonal BCR signaling proficient CLL subpopulation characterized by high CD20 levels. Leukemia 2018;32:2028–31.
Seda V, Vojackova E, Ondrisova L, Kostalova L, Sharma S, Loja T, et al. FoxO1-GAB1 axis regulates homing capacity and tonic AKT activity in chronic lymphocytic leukemia. Blood 2021;138:758–72.
Yang Q, Chen LS, Ha MJ, Do KA, Neelapu SS, Gandhi V. Idelalisib impacts cell growth through inhibiting translation-regulatory mechanisms in mantle cell lymphoma. Clin Cancer Res. 2017;23:181–92.
Wick MJ, Ramos FJ, Chen H, Quon MJ, Dong LQ, Liu F. Mouse 3-phosphoinositide-dependent protein kinase-1 undergoes dimerization and trans-phosphorylation in the activation loop. J Biol Chem. 2003;278:42913–9.
Mora A, Komander D, van Aalten DM, Alessi DR. PDK1, the master regulator of AGC kinase signal transduction. Semin Cell Dev Biol. 2004;15:161–70.
Currie RA, Walker KS, Gray A, Deak M, Casamayor A, Downes CP, et al. Role of phosphatidylinositol 3,4,5-trisphosphate in regulating the activity and localization of 3-phosphoinositide-dependent protein kinase-1. Biochem J. 1999;337:575–83.
Balendran A, Casamayor A, Deak M, Paterson A, Gaffney P, Currie R, et al. PDK1 acquires PDK2 activity in the presence of a synthetic peptide derived from the carboxyl terminus of PRK2. Curr Biol. 1999;9:393–404.
Biondi RM, Cheung PC, Casamayor A, Deak M, Currie RA, Alessi DR. Identification of a pocket in the PDK1 kinase domain that interacts with PIF and the C-terminal residues of PKA. EMBO J. 2000;19:979–88.
Collins BJ, Deak M, Arthur JS, Armit LJ, Alessi DR. In vivo role of the PIF-binding docking site of PDK1 defined by knock-in mutation. EMBO J. 2003;22:4202–11.
Acknowledgements
We thank all the CLL patients who participated in this study. This work was supported partly by a research fund from the National Cancer Institute (CA170006) and a Presbyterian Health Foundation (C5126601) Bridge grant to AKG. We also acknowledge the support received from the NCI Cancer Center Support Grant (P30 CA225520). In addition, this study was also supported in part by the Oklahoma Tobacco Settlement Endowment Trust awarded to the University of Oklahoma//Stephenson Cancer Center. JRB was supported by NIH R01 CA 213442.
Author information
Authors and Affiliations
Contributions
MKM, HM, GPM, MTM and SF performed experiments and collected data. MKM created the figures and table. SKV and JHC edited the manuscript. JRB provided clinical samples with relevant information, and edited the manuscript. AKG conceived and supervised the project, designed the research, analyzed data, and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
JRB has served as a consultant for Abbvie, Acerta, Astra-Zeneca, Beigene, Catapult, Dynamo Therapeutics, Eli Lilly, Juno/Celgene, Kite, MEI Pharma, Nextcea, Novartis, Octapharma, Pfizer, Rigel, Sunesis, TG Therapeutics, Verastem; received honoraria from Janssen; received research funding from Gilead, Loxo, Sun and Verastem; and served on data safety monitoring committees for Invectys. All other authors declare that there are no competing financial interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Mamidi, M.K., Mahmud, H., Maiti, G.P. et al. Idelalisib activates AKT via increased recruitment of PI3Kδ/PI3Kβ to BCR signalosome while reducing PDK1 in post-therapy CLL cells. Leukemia 36, 1806–1817 (2022). https://doi.org/10.1038/s41375-022-01595-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-022-01595-0