Abstract
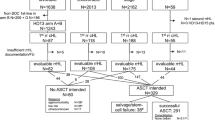
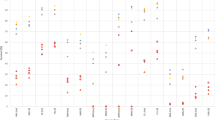
Data on the spectrum of second malignant neoplasms (SMNs) after primary childhood non-Hodgkin’s lymphoma (NHL) are scarce. One-hundred-and-eighty-nine NHL patients diagnosed in a 30 years period of 1980–2010 developing an SMN were retrieved from 19 members of the European Intergroup for Childhood NHL and/or the international Berlin-Frankfurt-Münster Study Group. Five subgroups of SMNs were identified: (1) myeloid neoplasms (n = 43; 23%), (2) lymphoid neoplasms (n = 51; 27%), (3) carcinomas (n = 48; 25%), (4) central nervous system (CNS) tumors (n = 19; 10%), and (5) “other” SMNs (n = 28; 15%). In 37 patients (20%) preexisting disorders were reported with 90% having any kind of cancer predisposition syndrome (CPS). For the 189 primary NHL patients, 5-year overall survival (OS) after diagnosis of an SMN was 56 ± 4%, being worst for patients with preexisting disorders at 28 ± 8%. Five-year OS rates were 38 ± 8%, 59 ± 7%, 79 ± 8%, 34 ± 12%, and 62 ± 11%, respectively, for patients with myeloid and lymphoid neoplasms, carcinomas, CNS tumors, and “other” SMNs (p < 0.0001). Patients with SMNs after childhood NHL having a reported CPS, mostly mismatch repair disorders, carried a very poor prognosis. Moreover, although outcome was favorable in some subtypes of SMNs after childhood NHL (carcinomas, lymphoid neoplasms), other SMNs such as myeloid neoplasms and CNS tumors had a dismal prognosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Minard-Colin V, Brugieres L, Reiter A, Cairo MS, Gross TG, Woessmann W, et al. Non-Hodgkin lymphoma in children and adolescents: progress through effective collaboration, current knowledge, and challenges ahead. J Clin Oncol. 2015;33:2963–74.
Attarbaschi A, Carraro E, Abla O, Barzilai-Birenboim S, Bomken S, Brugieres L, et al. Non-Hodgkin lymphoma and pre-existing conditions: spectrum, clinical characteristics and outcome in 213 children and adolescents. Haematologica. 2016;101:1581–91.
Bright CJ, Reulen RC, Winter DL, Stark DP, McCabe MG, Edgar AB, et al. Risk of subsequent primary neoplasms in survivors of adolescent and young adult cancer (Teenage and Young Adult Cancer Survivor Study): a population-based, cohort study. Lancet Oncol. 2019;20:531–45.
Leung W, Sandlund JT, Hudson MM, Zhou Y, Hancock ML, Zhu Y, et al. Second malignancy after treatment of childhood non-Hodgkin lymphoma. Cancer. 2001;92:1959–66.
Maule M, Scelo G, Pastore G, Brennan P, Hemminki K, Tracey E, et al. Risk of second malignant neoplasms after childhood leukemia and lymphoma: an international study. J Natl Cancer Inst. 2007;99:790–800.
Meadows AT, Friedman DL, Neglia JP, Mertens AC, Donaldson SS, Stovall M, et al. Second neoplasms in survivors of childhood cancer: findings from the Childhood Cancer Survivor Study cohort. J Clin Oncol. 2009;27:2356–62.
Kaatsch P, Reinisch I, Spix C, Berthold F, Janka-Schaub G, Mergenthaler A, et al. Case-control study on the therapy of childhood cancer and the occurrence of second malignant neoplasms in Germany. Cancer Causes Control. 2009;20:965–80.
Cardous-Ubbink MC, Heinen RC, Bakker PJ, van den Berg H, Oldenburger F, Caron HN, et al. Risk of second malignancies in long-term survivors of childhood cancer. Eur J Cancer. 2007;43:351–62.
Neglia JP, Friedman DL, Yasui Y, Mertens AC, Hammond S, Stovall M, et al. Second malignant neoplasms in five-year survivors of childhood cancer: childhood cancer survivor study. J Natl Cancer Inst. 2001;93:618–29.
Bluhm EC, Ronckers C, Hayashi RJ, Neglia JP, Mertens AC, Stovall M, et al. Cause-specific mortality and second cancer incidence after non-Hodgkin lymphoma: a report from the Childhood Cancer Survivor Study. Blood. 2008;111:4014–21.
Zhang J, Walsh MF, Wu G, Edmonson MN, Gruber TA, Easton J, et al. Germline mutations in predisposition genes in pediatric cancer. N Engl J Med. 2015;373:2336–46.
Ripperger T, Schlegelberger B. Acute lymphoblastic leukemia and lymphoma in the context of constitutional mismatch repair deficiency syndrome. Eur J Med Genet. 2016;59:133–42.
Ehrhardt MJ, Chen Y, Sandlund JT, Bluhm EC, Hayashi RJ, Becktell K, et al. Late health outcomes after contemporary Lymphome Malin de Burkitt Therapy for mature B-cell non-Hodgkin lymphoma: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2019;37:2556–70.
Ehrhardt MJ, Sandlund JT, Zhang N, Liu W, Ness KK, Bhakta N, et al. Late outcomes of adult survivors of childhood non-Hodgkin lymphoma: a report from the St. Jude Lifetime Cohort Study. Pediatr Blood Cancer. 2017;64:1–10.
Juskevicius D, Dirnhofer S, Tzankov A. Genetic background and evolution of relapses in aggressive B-cell lymphomas. Haematologica. 2017;102:1139–49.
Burkhardt B, Reiter A, Landmann E, Lang P, Lassay L, Dickerhoff R, et al. Poor outcome for children and adolescents with progressive disease or relapse of lymphoblastic lymphoma: a report from the berlin-frankfurt-muenster group. J Clin Oncol. 2009;27:3363–9.
Rigaud C, Auperin A, Jourdain A, Haouy S, Couec ML, Aladjidi N, et al. Outcome of relapse in children and adolescents with B-cell non-Hodgkin lymphoma and mature acute leukemia: a report from the French LMB study. Pediatr Blood Cancer. 2019;66:e27873.
Woessmann W, Zimmermann M, Meinhardt A, Mueller S, Hauch H, Knorr F, et al. Progressive or relapsed Burkitt lymphoma or leukemia in children and adolescents after BFM-type first-line therapy. Blood. 2020:1124–32.
Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J, et al. The World Health Organization classification of neoplasms of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting—Airlie House, Virginia, November, 1997. Hematol J. 2000;1:53–66.
Harris NL, Jaffe ES, Stein H, Banks PM, Chan JK, Cleary ML, et al. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood. 1994;84:1361–92.
Stansfeld AG, Diebold J, Noel H, Kapanci Y, Rilke F, Kelenyi G, et al. Updated Kiel classification for lymphomas. Lancet. 1988;1:292–3.
Lennert K. Morphology and classification of malignant lymphomas and so-called reticuloses. Acta Neuropathol Suppl. 1975;Suppl 6:1–16.
Lennert K, Stein H, Kaiserling E. Cytological and functional criteria for the classification of malignant lymphomata. Br J Cancer Suppl. 1975;2:29–43.
Murphy SB. Classification, staging and end results of treatment of childhood non-Hodgkin’s lymphomas: dissimilarities from lymphomas in adults. Semin Oncol. 1980;7:332–9.
Reiter A, Schrappe M, Ludwig WD, Tiemann M, Parwaresch R, Zimmermann M, et al. Intensive ALL-type therapy without local radiotherapy provides a 90% event-free survival for children with T-cell lymphoblastic lymphoma: a BFM group report. Blood 2000;95:416–21.
Reiter A, Schrappe M, Parwaresch R, Henze G, Muller-Weihrich S, Sauter S, et al. Non-Hodgkin’s lymphomas of childhood and adolescence: results of a treatment stratified for biologic subtypes and stage—a report of the Berlin-Frankfurt-Munster Group. J Clin Oncol. 1995;13:359–72.
Reiter A, Schrappe M, Tiemann M, Ludwig WD, Yakisan E, Zimmermann M, et al. Improved treatment results in childhood B-cell neoplasms with tailored intensification of therapy: a report of the Berlin-Frankfurt-Munster Group Trial NHL-BFM 90. Blood. 1999;94:3294–306.
Reiter A, Schrappe M, Tiemann M, Parwaresch R, Zimmermann M, Yakisan E, et al. Successful treatment strategy for Ki-1 anaplastic large-cell lymphoma of childhood: a prospective analysis of 62 patients enrolled in three consecutive Berlin-Frankfurt-Munster group studies. J Clin Oncol. 1994;12:899–908.
Burkhardt B, Woessmann W, Zimmermann M, Kontny U, Vormoor J, Doerffel W, et al. Impact of cranial radiotherapy on central nervous system prophylaxis in children and adolescents with central nervous system-negative stage III or IV lymphoblastic lymphoma. J Clin Oncol. 2006;24:491–9.
Woessmann W, Seidemann K, Mann G, Zimmermann M, Burkhardt B, Oschlies I, et al. The impact of the methotrexate administration schedule and dose in the treatment of children and adolescents with B-cell neoplasms: a report of the BFM Group Study NHL-BFM95. Blood. 2005;105:948–58.
Pillon M, Arico M, Mussolin L, Carraro E, Conter V, Sala A, et al. Long-term results of the AIEOP LNH-97 protocol for childhood lymphoblastic lymphoma. Pediatr Blood Cancer. 2015;62:1388–94.
Pillon M, Gregucci F, Lombardi A, Santoro N, Piglione M, Sala A, et al. Results of AIEOP LNH-97 protocol for the treatment of anaplastic large cell lymphoma of childhood. Pediatr Blood Cancer. 2012;59:828–33.
Pillon M, Piglione M, Garaventa A, Conter V, Giuliano M, Arcamone G, et al. Long-term results of AIEOP LNH-92 protocol for the treatment of pediatric lymphoblastic lymphoma: a report of the Italian Association of Pediatric Hematology and Oncology. Pediatr Blood Cancer. 2009;53:953–9.
Atra A, Gerrard M, Hobson R, Imeson JD, Ashley S, Pinkerton CR. Improved cure rate in children with B-cell acute lymphoblastic leukaemia (B-ALL) and stage IV B-cell non-Hodgkin’s lymphoma (B-NHL)—results of the UKCCSG 9003 protocol. Br J Cancer. 1998;77:2281–5.
Atra A, Imeson JD, Hobson R, Gerrard M, Hann IM, Eden OB, et al. Improved outcome in children with advanced stage B-cell non-Hodgkin’s lymphoma (B-NHL): results of the United Kingdom Children Cancer Study Group (UKCCSG) 9002 protocol. Br J Cancer. 2000;82:1396–402.
Gerrard M, Cairo MS, Weston C, Auperin A, Pinkerton R, Lambilliote A, et al. Excellent survival following two courses of COPAD chemotherapy in children and adolescents with resected localized B-cell non-Hodgkin’s lymphoma: results of the FAB/LMB 96 international study. Br J Haematol. 2008;141:840–7.
Gerrard M, Waxman IM, Sposto R, Auperin A, Perkins SL, Goldman S, et al. Outcome and pathologic classification of children and adolescents with mediastinal large B-cell lymphoma treated with FAB/LMB96 mature B-NHL therapy. Blood. 2013;121:278–85.
Le Deley MC, Rosolen A, Williams DM, Horibe K, Wrobel G, Attarbaschi A, et al. Vinblastine in children and adolescents with high-risk anaplastic large-cell lymphoma: results of the randomized ALCL99-vinblastine trial. J Clin Oncol. 2010;28:3987–93.
Patte C, Auperin A, Gerrard M, Michon J, Pinkerton R, Sposto R, et al. Results of the randomized international FAB/LMB96 trial for intermediate risk B-cell non-Hodgkin lymphoma in children and adolescents: it is possible to reduce treatment for the early responding patients. Blood. 2007;109:2773–80.
Tsurusawa M, Mori T, Kikuchi A, Mitsui T, Sunami S, Kobayashi R, et al. Improved treatment results of children with B-cell non-Hodgkin lymphoma: a report from the Japanese Pediatric Leukemia/Lymphoma Study Group B-NHL03 study. Pediatr Blood Cancer. 2014;61:1215–21.
Bergeron C, Coze C, Segura C, Pacquement H, Gandemer V, Ducassou S, et al. Treatment of childhood T-cell lymphoblastic lymphoma-long-term results of the SFOP LMT96 Trial. Pediatr Blood Cancer. 2015;62:2150–6.
Brugieres L, Le Deley MC, Rosolen A, Williams D, Horibe K, Wrobel G, et al. Impact of the methotrexate administration dose on the need for intrathecal treatment in children and adolescents with anaplastic large-cell lymphoma: results of a randomized trial of the EICNHL Group. J Clin Oncol. 2009;27:897–903.
Goldman S, Smith L, Anderson JR, Perkins S, Harrison L, Geyer MB, et al. Rituximab and FAB/LMB 96 chemotherapy in children with Stage III/IV B-cell non-Hodgkin lymphoma: a Children’s Oncology Group report. Leukemia. 2013;27:1174–7.
Rosolen A, Pillon M, Garaventa A, Burnelli R, d’Amore ES, Giuliano M, et al. Anaplastic large cell lymphoma treated with a leukemia-like therapy: report of the Italian Association of Pediatric Hematology and Oncology (AIEOP) LNH-92 protocol. Cancer. 2005;104:2133–40.
Seidemann K, Tiemann M, Schrappe M, Yakisan E, Simonitsch I, Janka-Schaub G, et al. Short-pulse B-non-Hodgkin lymphoma-type chemotherapy is efficacious treatment for pediatric anaplastic large cell lymphoma: a report of the Berlin-Frankfurt-Munster Group Trial NHL-BFM 90. Blood. 2001;97:3699–706.
Landmann E, Burkhardt B, Zimmermann M, Meyer U, Woessmann W, Klapper W, et al. Results and conclusions of the European Intergroup EURO-LB02 trial in children and adolescents with lymphoblastic lymphoma. Haematologica. 2017;102:2086–96.
Reiter A, Schrappe M, Ludwig WD, Lampert F, Harbott J, Henze G, et al. Favorable outcome of B-cell acute lymphoblastic leukemia in childhood: a report of three consecutive studies of the BFM group. Blood. 1992;80:2471–8.
Amylon MD, Shuster J, Pullen J, Berard C, Link MP, Wharam M, et al. Intensive high-dose asparaginase consolidation improves survival for pediatric patients with T cell acute lymphoblastic leukemia and advanced stage lymphoblastic lymphoma: a Pediatric Oncology Group study. Leukemia. 1999;13:335–42.
Stary J, Zimmermann M, Campbell M, Castillo L, Dibar E, Donska S, et al. Intensive chemotherapy for childhood acute lymphoblastic leukemia: results of the randomized intercontinental trial ALL IC-BFM 2002. J Clin Oncol. 2014;32:174–84.
Uyttebroeck A, Suciu S, Laureys G, Robert A, Pacquement H, Ferster A, et al. Treatment of childhood T-cell lymphoblastic lymphoma according to the strategy for acute lymphoblastic leukaemia, without radiotherapy: long term results of the EORTC CLG 58881 trial. Eur J Cancer. 2008;44:840–6.
Burkhardt B, Hermiston ML. Lymphoblastic lymphoma in children and adolescents: review of current challenges and future opportunities. Br J Haematol. 2019;185:1158–70.
Egan G, Goldman S, Alexander S. Mature B-NHL in children, adolescents and young adults: current therapeutic approach and emerging treatment strategies. Br J Haematol. 2019;185:1071–85.
Schmiegelow K, Levinsen MF, Attarbaschi A, Baruchel A, Devidas M, Escherich G, et al. Second malignant neoplasms after treatment of childhood acute lymphoblastic leukemia. J Clin Oncol. 2013;31:2469–76.
Bienemann K, Burkhardt B, Modlich S, Meyer U, Moricke A, Bienemann K, et al. Promising therapy results for lymphoid malignancies in children with chromosomal breakage syndromes (Ataxia teleangiectasia or Nijmegen-breakage syndrome): a retrospective survey. Br J Haematol. 2011;155:468–76.
Arico M, Mussolin L, Carraro E, Buffardi S, Santoro N, D’Angelo P, et al. Non-Hodgkin lymphoma in children with an associated inherited condition: a retrospective analysis of the Associazione Italiana Ematologia Oncologia Pediatrica (AIEOP). Pediatr Blood Cancer. 2015;62:1782–9.
Jongmans MC, Loeffen JL, Waanders E, Hoogerbrugge PM, Ligtenberg MJ, Kuiper RP, et al. Recognition of genetic predisposition in pediatric cancer patients: an easy-to-use selection tool. Eur J Med Genet. 2016;59:116–25.
Ripperger T, Bielack SS, Borkhardt A, Brecht IB, Burkhardt B, Calaminus G, et al. Childhood cancer predisposition syndromes-A concise review and recommendations by the Cancer Predisposition Working Group of the Society for Pediatric Oncology and Hematology. Am J Med Genet A. 2017;173:1017–37.
Wimmer K, Kratz CP, Vasen HF, Caron O, Colas C, Entz-Werle N, et al. Diagnostic criteria for constitutional mismatch repair deficiency syndrome: suggestions of the European consortium ‘care for CMMRD’ (C4CMMRD). J Med Genet. 2014;51:355–65.
Vasen HF, Ghorbanoghli Z, Bourdeaut F, Cabaret O, Caron O, Duval A, et al. Guidelines for surveillance of individuals with constitutional mismatch repair-deficiency proposed by the European Consortium “Care for CMMR-D” (C4CMMR-D). J Med Genet. 2014;51:283–93.
Acknowledgements
We thank all participating institutions and physicians for their support of the study. This EICNHL and i-BFM paper was written on behalf of the Berlin-Frankfurt-Münster (BFM) Study Group (Austria, Germany, Switzerland, Czech Republic), Associazione Italiana Ematologia e Oncologia Pediatrica (AIEOP), Société Française de Lutte contre les Cancers et Leucémies de l’Enfant (SFCE), United Kingdom Children’s Cancer and Leukemia Study Group (CCLG), Belgian Society of Pediatric Hematology and Oncology, Dutch Childhood Oncology Group (DCOG), Nordic Society of Pediatric Hematology and Oncology (NOPHO), Hungarian Pediatric Oncology Network, Slovenian Society of Hematology and Oncology, Japanese Pediatric Leukemia/Lymphoma Study Group (JPLSG), Hong Kong Pediatric Hematology and Oncology Study Group (HKPHOSG), Hellenic Society of Pediatric Hematology and Oncology (HeSHOP), Israel’s Society of Pediatric Hematology and Oncology, Spanish Society of Pediatric Hematology and Oncology (SEHOP), and two single institutions from Belarus (Minsk) and Russia (Moscow).
This work was supported by Cancer Research United Kingdom, the Deutsche Kinderkrebsstiftung (BB and WW), DKS 2014.11A/B, DKS 2016.24A/B (BFM Germany), the St. Anna Kinderkrebsforschung (AA, BFM Austria), the Czech Ministry of Health supported project for conceptual development of research organization 00064203 (EK, BFM Czech Republic), and the Ministry of Health, Labour and Welfare of Japan (TO, JPLSG).
Author information
Authors and Affiliations
Consortia
Contributions
AA and MP designed and planned the study; AA and MP wrote the paper; AA, EC, LR, and MP were in charge of data pooling, data checking, and statistical analysis; all other authors (MA, SBB, SB, LB, BB, FC, AC, AP, MC, AF, JJ, EK, JL, NM, KM, OM, TO, CR, AU, WW) as well as AA and MP were principal or co-investigators in their study groups and institutions, coordinated the national trials in their countries, provided study materials, and recruited patients. All authors read and approved the final version of the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Attarbaschi, A., Carraro, E., Ronceray, L. et al. Second malignant neoplasms after treatment of non-Hodgkin’s lymphoma—a retrospective multinational study of 189 children and adolescents. Leukemia 35, 534–549 (2021). https://doi.org/10.1038/s41375-020-0841-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-020-0841-x
This article is cited by
-
Characteristics and outcomes of children, adolescents and young adults with relapsed/refractory non-hodgkin lymphoma undergoing autologous stem cell transplant
BMC Cancer (2023)
-
Second malignant neoplasms in lymphomas, secondary lymphomas and lymphomas in metabolic disorders/diseases
Cell & Bioscience (2022)
-
Genomic abnormalities of TP53 define distinct risk groups of paediatric B-cell non-Hodgkin lymphoma
Leukemia (2022)
-
Risk and prognosis of secondary breast cancer after radiation therapy for non-Hodgkin lymphoma: a massive population-based analysis
Clinical and Translational Oncology (2022)