Abstract
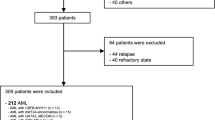
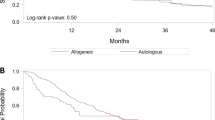
Hyperleukocytosis in acute myeloid leukemia (AML) is associated with inferior outcomes. There is limited high quality evidence to support the benefits of leukapheresis. We retrospectively collected data from patients with newly-diagnosed AML who presented with a white cell count (WBC) >50 × 109/L to 12 centers in the United States and Europe from 2006 to 2017 and received intensive chemotherapy. Logistic regression models estimated odds ratios for 30-day mortality and achievement of composite complete remission (CRc). Cox proportional hazard models estimated hazard ratios for overall survival (OS). Among 779 patients, clinical leukostasis was reported in 27%, and leukapheresis was used in 113 patients (15%). Thirty-day mortality was 16.7% (95% CI: 13.9–19.3%). Median OS was 12.6 months (95% CI: 11.5–14.9) among all patients, and 4.5 months (95% CI: 2.7–7.1) among those ≥65 years. Use of leukapheresis did not significantly impact 30-day mortality, achievement of CRc, or OS in multivariate analysis based on available data or in analysis based on multiple imputation. Among patients with investigator-adjudicated clinical leukostasis, there were statistically significant improvements in 30-day mortality and OS with leukapheresis in unadjusted analysis, but not in multivariate analysis. Given the significant resource use, cost, and potential complications of leukapheresis, randomized studies are needed to evaluate its value.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Rollig C, Ehninger G. How I treat hyperleukocytosis in acute myeloid leukemia. Blood. 2015;125:3246–52.
Greenwood MJ, Seftel MD, Richardson C, Barbaric D, Barnett MJ, Bruyere H, et al. Leukocyte count as a predictor of death during remission induction in acute myeloid leukemia. Leuk Lymphoma. 2006;47:1245–52.
Marbello L, Ricci F, Nosari AM, Turrini M, Nador G, Nichelatti M, et al. Outcome of hyperleukocytic adult acute myeloid leukaemia: a single-center retrospective study and review of literature. Leuk Res. 2008;32:1221–7.
Porcu P, Danielson CF, Orazi A, Heerema NA, Gabig TG, McCarthy LJ. Therapeutic leukapheresis in hyperleucocytic leukaemias: lack of correlation between degree of cytoreduction and early mortality rate. Br J Haematol. 1997;98:433–6.
De Santis GC, de Oliveira LC, Romano LG, Almeida Prado Bde P Jr, Simoes BP, Rego EM, et al. Therapeutic leukapheresis in patients with leukostasis secondary to acute myelogenous leukemia. J Clin Apher. 2011;26:181–5.
Porcu P, Cripe LD, Ng EW, Bhatia S, Danielson CM, Orazi A, et al. Hyperleukocytic leukemias and leukostasis: a review of pathophysiology, clinical presentation and management. Leuk Lymphoma. 2000;39:1–18.
Dohner H, Estey EH, Amadori S, Appelbaum FR, Buchner T, Burnett AK, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453–74.
Zuckerman T, Ganzel C, Tallman MS, Rowe JM. How I treat hematologic emergencies in adults with acute leukemia. Blood. 2012;120:1993–2002.
Bruserud O, Liseth K, Stamnesfet S, Cacic DL, Melve G, Kristoffersen E, et al. Hyperleukocytosis and leukocytapheresis in acute leukaemias: experience from a single centre and review of the literature of leukocytapheresis in acute myeloid leukaemia. Transfus Med. 2013;23:397–406.
Bug G, Anargyrou K, Tonn T, Bialleck H, Seifried E, Hoelzer D, et al. Impact of leukapheresis on early death rate in adult acute myeloid leukemia presenting with hyperleukocytosis. Transfusion. 2007;47:1843–50.
Giles FJ, Shen Y, Kantarjian HM, Korbling MJ, O’Brien S, Anderlini P, et al. Leukapheresis reduces early mortality in patients with acute myeloid leukemia with high white cell counts but does not improve long- term survival. Leuk Lymphoma. 2001;42:67–73.
Thiebaut A, Thomas X, Belhabri A, Anglaret B, Archimbaud E. Impact of pre-induction therapy leukapheresis on treatment outcome in adult acute myelogenous leukemia presenting with hyperleukocytosis. Ann Hematol. 2000;79:501–6.
Oberoi S, Lehrnbecher T, Phillips B, Hitzler J, Ethier MC, Beyene J, et al. Leukapheresis and low-dose chemotherapy do not reduce early mortality in acute myeloid leukemia hyperleukocytosis: a systematic review and meta-analysis. Leuk Res. 2014;38:460–8.
Berber I, Kuku I, Erkurt MA, Kaya E, Bag HG, Nizam I, et al. Leukapheresis in acute myeloid leukemia patients with hyperleukocytosis: a single center experience. Transfus Apher Sci. 2015;53:185–90.
Choi MH, Choe YH, Park Y, Nah H, Kim S, Jeong SH, et al. The effect of therapeutic leukapheresis on early complications and outcomes in patients with acute leukemia and hyperleukocytosis: a propensity score-matched study. Transfusion. 2018;58:208–16.
Chang MC, Chen TY, Tang JL, Lan YJ, Chao TY, Chiu CF, et al. Leukapheresis and cranial irradiation in patients with hyperleukocytic acute myeloid leukemia: no impact on early mortality and intracranial hemorrhage. Am J Hematol. 2007;82:976–80.
Inaba H, Fan Y, Pounds S, Geiger TL, Rubnitz JE, Ribeiro RC, et al. Clinical and biologic features and treatment outcome of children with newly diagnosed acute myeloid leukemia and hyperleukocytosis. Cancer. 2008;113:522–9.
Stahl M, Pine A, Hendrickson JE, Litzow MR, Luger SM, Stone RM, et al. Beliefs and practice patterns in hyperleukocytosis management in acute myeloid leukemia: a large U.S. web-based survey. Leuk Lymphoma. 2018;59:1–4.
Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–405.
Dohner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Buchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424–47.
Grimwade D, Hills RK, Moorman AV, Walker H, Chatters S, Goldstone AH, et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. 2010;116:354–65.
Breems DA, Van Putten WL, De Greef GE, Van Zelderen-Bhola SL, Gerssen-Schoorl KB, Mellink CH, et al. Monosomal karyotype in acute myeloid leukemia: a better indicator of poor prognosis than a complex karyotype. J Clin Oncol. 2008;26:4791–7.
Shallis RM, Stahl M, Wei W, et al. Patterns of care and clinical outcomes of patients with newly diagnosed acute myeloid leukemia presenting with hyperleukocytosis who do not receive intensive chemotherapy. Leuk Lymphoma. 2020;1–6. https://doi.org/10.1080/10428194.2020.1728753 [published online ahead of print, 26 Feb 2020].
Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–9.
Buuren Sv, Groothuis-Oudshroon K. Mice: multivariate imputation by chained equations in R. J Stat Softw. 2011;45:1–67.
Sorita A, Ahmed A, Starr SR, Thompson KM, Reed DA, Prokop L, et al. Off-hour presentation and outcomes in patients with acute myocardial infarction: systematic review and meta-analysis. BMJ. 2014;348:f7393.
Kostis WJ, Demissie K, Marcella SW, Shao YH, Wilson AC, Moreyra AE, et al. Weekend versus weekday admission and mortality from myocardial infarction. N Engl J Med. 2007;356:1099–109.
Janszky I, Ahnve S, Ljung R. Weekend versus weekday admission and stroke outcome in Sweden from 1968 to 2005. Stroke. 2007;38:e94. author reply e95.
Reeves MJ, Smith E, Fonarow G, Hernandez A, Pan W, Schwamm LH, et al. Off-hour admission and in-hospital stroke case fatality in the get with the guidelines-stroke program. Stroke. 2009;40:569–76.
Daver N, Kantarjian H, Marcucci G, Pierce S, Brandt M, Dinardo C, et al. Clinical characteristics and outcomes in patients with acute promyelocytic leukaemia and hyperleucocytosis. Br J Haematol. 2015;168:646–53.
Acknowledgements
AZ is a Leukemia and Lymphoma Society Scholar in Clinical Research and is also supported by a NCI’s Cancer Clinical Investigator Team Leadership Award (CCITLA). Research reported in this publication was in part supported by the National Cancer Institute of the National Institutes of Health under Award Number P30 CA016359. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We would like to acknowledge all the patients who contributed data for this study, and the Frederick A. DeLuca Foundation for supporting the statistical analyses. MS is supported by the MSKCC Clinical Scholars T32 Program under award number 2T32 CA009512-31.
Author information
Authors and Affiliations
Contributions
Conception and design: MS, RS, WW, and AMZ. Provision of study materials or patients: all authors. Collection and assembly of data: all authors. Data analysis and interpretation: MS, RS, WW, and AMZ. Paper writing: all authors. Paper critical revision: all authors. Final approval of paper: all authors. Accountable for all aspects of the work: all authors.
Corresponding author
Ethics declarations
Conflict of interest
MS: no relevant financial relationship(s) to disclose. RMS: no relevant financial relationship(s) to disclose. WW: no relevant financial relationship(s) to disclose. PM: Daiichi Sankyo: Consultancy and Speakers Bureau. Novartis: Research Funding and Speakers Bureau. EL: No relevant financial relationship(s) to disclose. JN: no relevant financial relationship(s) to disclose. VRB: consulting fees: CSL Behring, Agios, Abbvie, Partner therapeutics and Incyte. Research funding: Jazz, Incyte, Tolero Pharmaceuticals, Inc, and National Marrow Donor Program. MAS: no relevant financial relationship(s) to disclose. ATF: consulting/advisory board—Celgene, Takeda, Abbvie, Forty seven, NewLink, Trovagene, Pfizer, Daiichi Sankyo, Astellas, Amphivena. Clinical trial funding—Celgene and Agios. HK: no relevant financial relationship(s) to disclose. SL: no relevant financial relationship(s) to disclose. IK: Teva: Speakers Bureau. GJR: AbbVie: Consultancy. Amphivena Therapeutics: Consultancy. Argenx: Consultancy. Astex Pharmaceuticals: Consultancy. Bayer: Consultancy. Celgene Corporation: Consultancy. Celltrion: Consultancy. Daiichi Sankyo: Consultancy. Bayer: Consultancy. Eisai: Consultancy. Janssen Pharmaceuticals: Consultancy. Jazz Pharmaceuticals: Consultancy. Novartis: Consultancy. Daiichi Sankyo: Consultancy. Orsenix: Consultancy. Otsuka: Consultancy. Eisai: Consultancy. Pfizer: Consultancy. Roche/Genentech: Consultancy. Sandoz: Consultancy. Cellectis: Research Funding. Orsenix: Consultancy. TC: Celgene: Consultancy, Membership on an entity’s Board of Directors or advisory committees and Speakers. Bureau. Menarini: Consultancy. Jazz Pharma: Consultancy, Membership on an entity’s Board of Directors or advisory committees and Speakers Bureau. Amgen: Membership on an entity’s Board of Directors or advisory committees and Speakers Bureau. AbbVie: Membership on an entity’s Board of Directors or advisory committees and Speakers Bureau. Sanofi: Speakers Bureau. Pfizer: Speakers Bureau. DMC: no relevant financial relationship(s) to disclose. ER: no relevant financial relationship(s) to disclose. UG: Celgene: Honoraria, Research Funding and Consultancy. Novartis: Honoraria and Research Funding. Janssen: Honoraria. JMU: no relevant financial relationship(s) to disclose. SM: Aplastic Anemia & MDS International Foundation in Joint Partnership with Cleveland Clinic Taussig Cancer Institute: Honoraria. BioPharm Communications: Consultancy. Bristol Myers Squib: Honoraria and Speakers Bureau. LEK Consulting: Consultancy and Honoraria. Novartis: Consultancy, Membership on an entity’s Board of Directors or advisory committees and Research Funding. Pfizer: Honoraria. Projects in Knowledge: Honoraria. Takeda: Membership on an entity’s Board of Directors or advisory committees. AMB: Takeda: Research Funding. Celgene: Consultancy and Research Funding. Novartis: Research Funding. AMM: no relevant financial relationship(s) to disclose CMM: no relevant financial relationship(s) to disclose. EKR: Incyte: Consultancy and Speakers Bureau. Celgene: Consultancy, Other: Travel, Accommodations, Expenses and Speakers Bureau. Pfizer: Consultancy and Research Funding. Novartis: Consultancy, Other: Travel, Accommodations, Expenses, Research Funding and Speakers Bureau. ARIAD Pharmaceuticals: Speakers Bureau. Astellas Pharma: Research Funding. Bristol Myers Squibb: Research Funding. NS Pharma: Research Funding. RRV: no relevant financial relationship(s) to disclose. RI: no relevant financial relationship(s) to disclose. BB: No relevant financial relationship(s) to disclose. FR: no relevant financial relationship(s) to disclose. MT: no relevant financial relationship(s) to disclose. EGAC: no relevant financial relationship(s) to disclose. ER: no relevant financial relationship(s) to disclose. BY: no relevant financial relationship(s) to disclose. IC: no relevant financial relationship(s) to disclose. NAP: Agios Pharmaceuticals: Consultancy and Honoraria. Astellas Pharma: Research Funding. Blueprint Medicines: Consultancy and Honoraria. Incyte: Consultancy and Honoraria. Novartis: Consultancy and Honoraria. Boehringer-Ingelheim: Research Funding. Daiichi Sankyo: Research Funding. Sunesis Pharmaceuticals: Research Funding. Celator: Research Funding. Pfizer: Research Funding, Consultancy and Honoraria. Astex Pharmaceuticals: Research Funding. Celgene: Research Funding, Consultancy and Honoraria. Genentech: Research Funding. AI Therapeutics: Research Funding. Samus Therapeutics: Research Funding. Arog Pharmaceuticals: Research Funding. Kartos Therapeutics: Research Funding. JPB: no relevant financial relationship(s) to disclose. SDG: Celgene: Consultancy and Research Funding. AMZ received research funding from Celgene, Acceleron, Abbvie, Otsuka, Pfizer, Medimmune/AstraZeneca, Boehringer-Ingelheim, Trovagene, Incyte, Takeda, Novartis, Aprea, Astex, and ADC Therapeutics. AMZ had a consultancy with and received honoraria from AbbVie, Otsuka, Pfizer, Celgene, Jazz, Incyte, Agios, Boehringer-Ingelheim, Novartis, Acceleron, Astellas, Daiichi Sankyo, Cardinal Health, Seattle Genetics, BeyondSpring, Trovagene, Takeda, Ionis, and Epizyme. AMZ received travel support for meetings from Pfizer, Novartis, and Trovagene. None of these relationships were related to the development of this paper.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Stahl, M., Shallis, R.M., Wei, W. et al. Management of hyperleukocytosis and impact of leukapheresis among patients with acute myeloid leukemia (AML) on short- and long-term clinical outcomes: a large, retrospective, multicenter, international study. Leukemia 34, 3149–3160 (2020). https://doi.org/10.1038/s41375-020-0783-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-020-0783-3
This article is cited by
-
Krebspatienten in der Notaufnahme
Medizinische Klinik - Intensivmedizin und Notfallmedizin (2024)
-
Benefits of dexamethasone on early outcomes in patients with acute myeloid leukemia with hyperleukocytosis: a propensity score matched analysis
Annals of Hematology (2023)
-
Genetic mutations and leukapheresis in acute myeloid leukemia: is there a link?
Annals of Hematology (2023)
-
Molecular dissection of a hyper-aggressive CBFB-MYH11/FLT3-ITD–positive acute myeloid leukemia
Journal of Translational Medicine (2022)
-
Genomic landscape of hyperleukocytic acute myeloid leukemia
Blood Cancer Journal (2022)