Abstract
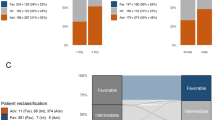
Recently, mRNA-expression signature enriched in LSCs was used to create a 17-gene leukemic stem cell (LSC17) score predictive of prognosis in adult AML. By fitting a Cox-LASSO regression model to the clinical outcome and gene-expression levels of LSC enriched genes in 163 pediatric participants of the AML02 multi-center clinical trial (NCT00136084), we developed a six-gene LSC score of prognostic value in pediatric AML (pLSC6). In the AML02 cohort, the 5-year event-free survival (EFS) of patients within low-pLSC6 group (n = 97) was 78.3 (95% CI = 70.5–86.9%) as compared with 34.5(95% CI = 24.7–48.2 %) in patients within high-pLSC6 group (n = 66 subjects), p < 0.00001. pLSC6 remained significantly associated with EFS and overall survival (OS) after adjusting for induction 1-MRD status, risk-group, FLT3-status, WBC-count at diagnosis and age. pLSC6 formula developed in the AML02 cohort was validated in the pediatric AML-TARGET project data (n = 205), confirming its prognostic value in both single-predictor and multiple-predictor Cox regression models. In both cohorts, pLSC6 predicted outcome of transplant patients, suggesting it as a useful criterion for transplant referrals. Our results suggest that pLSC6 score holds promise in redefining initial risk-stratification and identifying poor risk AML thereby providing guidance for developing novel treatment strategies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
16 April 2020
A Correction to this paper has been published: https://doi.org/10.1038/s41375-020-0822-0
References
Terwijn M, Zeijlemaker W, Kelder A, Rutten AP, Snel AN, Scholten WJ, et al. Leukemic stem cell frequency: a strong biomarker for clinical outcome in acute myeloid leukemia. PLoS One. 2014;9:e107587.
van Rhenen A, Feller N, Kelder A, Westra AH, Rombouts E, Zweegman S, et al. High stem cell frequency in acute myeloid leukemia at diagnosis predicts high minimal residual disease and poor survival. Clin Cancer Res. 2005;11:6520–7.
Ng SW, Mitchell A, Kennedy JA, Chen WC, McLeod J, Ibrahimova N, et al. A 17-gene stemness score for rapid determination of risk in acute leukaemia. Nature. 2016;540:433–7.
de Rooij JD, Zwaan CM, van den Heuvel-Eibrink M. Pediatric AML: from biology to clinical management. J Clin Med. 2015;4:127–49.
Rubnitz JE, Inaba H, Dahl G, Ribeiro RC, Bowman WP, Taub J, et al. Minimal residual disease-directed therapy for childhood acute myeloid leukaemia: results of the AML02 multicentre trial. Lancet Oncol. 2010;11:543–52.
Gamis AS, Alonzo TA, Meshinchi S, Sung L, Gerbing RB, Raimondi SC, et al. Gemtuzumab ozogamicin in children and adolescents with de novo acute myeloid leukemia improves event-free survival by reducing relapse risk: results from the randomized phase III Children’s Oncology Group trial AAML0531. J Clin Oncol. 2014;32:3021–32.
Cooper TM, Franklin J, Gerbing RB, Alonzo TA, Hurwitz C, Raimondi SC, et al. AAML03P1, a pilot study of the safety of gemtuzumab ozogamicin in combination with chemotherapy for newly diagnosed childhood acute myeloid leukemia: a report from the Children’s Oncology Group. Cancer. 2012;118:761–9.
Ross ME, Mahfouz R, Onciu M, Liu HC, Zhou X, Song G, et al. Gene expression profiling of pediatric acute myelogenous leukemia. Blood. 2004;104:3679–87.
Heinze G, Dunkler D. Avoiding infinite estimates of time-dependent effects in small-sample survival studies. Stat Med. 2008;27:6455–69.
Heinze G, Schemper M. A solution to the problem of monotone likelihood in Cox regression. Biometrics. 2001;57:114–9.
Efron B, Tibshirani R. Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Stat Sci. 1986;1.
Richard-Carpentier G, Sauvageau G. Bringing a leukemic stem cell gene signature into clinics: are we there yet? Cell Stem Cell. 2017;20:300–1.
Eppert K, Takenaka K, Lechman ER, Waldron L, Nilsson B, van Galen P, et al. Stem cell gene expression programs influence clinical outcome in human leukemia. Nat Med. 2011;17:1086–93.
de Jonge HJ, Woolthuis CM, Vos AZ, Mulder A, van den Berg E, Kluin PM, et al. Gene expression profiling in the leukemic stem cell-enriched CD34+ fraction identifies target genes that predict prognosis in normal karyotype AML. Leukemia. 2011;25:1825–33.
Gentles AJ, Plevritis SK, Majeti R, Alizadeh AA. Association of a leukemic stem cell gene expression signature with clinical outcomes in acute myeloid leukemia. JAMA. 2010;304:2706–15.
Duployez N, Marceau-Renaut A, Villenet C, Petit A, Rousseau A, Ng SWK, et al. The stem cell-associated gene expression signature allows risk stratification in pediatric acute myeloid leukemia. Leukemia. 2018;33:348–57.
Niederwieser C, Kohlschmidt J, Volinia S, Whitman SP, Metzeler KH, Eisfeld AK, et al. Prognostic and biologic significance of DNMT3B expression in older patients with cytogenetically normal primary acute myeloid leukemia. Leukemia. 2015;29:567–75.
Lamba JK, Cao X, Raimondi SC, Rafiee R, Downing JR, Lei S, et al. Integrated epigenetic and genetic analysis identifies markers of prognostic significance in pediatric acute myeloid leukemia. Oncotarget. 2018;9:26711–23.
Pabst C, Bergeron A, Lavallee VP, Yeh J, Gendron P, Norddahl GL, et al. GPR56 identifies primary human acute myeloid leukemia cells with high repopulating potential in vivo. Blood. 2016;127:2018–27.
Daria D, Kirsten N, Muranyi A, Mulaw M, Ihme S, Kechter A, et al. GPR56 contributes to the development of acute myeloid leukemia in mice. Leukemia. 2016;30:1734–41.
Saito Y, Morishita K. Maintenance of leukemic and normal hematopoietic stem cells in bone marrow niches by EVI1-regulated GPR56. Rinsho Ketsueki. 2015;56:375–83.
Laszlo GS, Ries RE, Gudgeon CJ, Harrington KH, Alonzo TA, Gerbing RB, et al. High expression of suppressor of cytokine signaling-2 predicts poor outcome in pediatric acute myeloid leukemia: a report from the Children’s Oncology Group. Leuk Lymphoma. 2014;55:2817–21.
Chen T, Lee TR, Liang WG, Chang WS, Lyu PC. Identification of trypsin-inhibitory site and structure determination of human SPINK2 serine proteinase inhibitor. Proteins. 2009;77:209–19.
Deng DX, Zhu HH, Liu YR, Chang YJ, Ruan GR, Jia JS, et al. Minimal residual disease detected by multiparameter flow cytometry is complementary to genetics for risk stratification treatment in acute myeloid leukemia with biallelic CEBPA mutations. Leuk Lymphoma. 2019;60:2181–9.
Moors I, Vandepoele K, Philippe J, Deeren D, Selleslag D, Breems D, et al. Clinical implications of measurable residual disease in AML: review of current evidence. Crit Rev Oncol Hematol. 2019;133:142–8.
Acknowledgements
We gratefully acknowledge funding from ALSAC, University of Florida-Opportunity funds and NIH grant R01-CA132946 (Lamba and Pounds). We thank Dr. Dario Campana and Coustan-Smith for minimal residual disease (MRD) data and Dr. Soheil Meshinchi for insightful comments.
Author information
Authors and Affiliations
Contributions
Study concept and design: JKL, AHE, and SP; Acquisition, analysis, and interpretation of data: All authors; Drafting manuscript: JKL, AHE, and SP; Critical revision of manuscript for important intellectual content: All authors; Statistical analysis: SP, XC, AHE, and YF. JKL and SP had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This research was supported by NIH R01CA132946 and University of Florida, Opportunity funds.
This work was previously presented in the oral session at 2018 Annual American Society of Hematology meeting, Dec 2018, San Diego, California, USA.
Supplementary information
Rights and permissions
About this article
Cite this article
Elsayed, A.H., Rafiee, R., Cao, X. et al. A six-gene leukemic stem cell score identifies high risk pediatric acute myeloid leukemia. Leukemia 34, 735–745 (2020). https://doi.org/10.1038/s41375-019-0604-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-019-0604-8
This article is cited by
-
Development and validation of a promising 5-gene prognostic model for pediatric acute myeloid leukemia
Molecular Biomedicine (2024)
-
Cellular hierarchy insights reveal leukemic stem-like cells and early death risk in acute promyelocytic leukemia
Nature Communications (2024)
-
Preclinical efficacy of targeting epigenetic mechanisms in AML with 3q26 lesions and EVI1 overexpression
Leukemia (2024)
-
A new genomic framework to categorize pediatric acute myeloid leukemia
Nature Genetics (2024)
-
Single-cell analysis reveals altered tumor microenvironments of relapse- and remission-associated pediatric acute myeloid leukemia
Nature Communications (2023)