Abstract
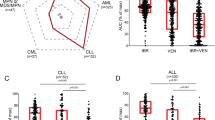
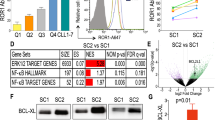
Recently, several small molecule drugs were approved for treatment of chronic lymphocytic leukemia (CLL), significantly improving patient management. However, knowledge about how to combine these therapies for optimal effects and what patients will best benefit from them is lacking. Here, we show that drug synergies can be identified by single cell signaling analyses. We investigated the effects of idelalisib, ibrutinib, and venetoclax on 35 protein epitopes by phospho flow in CLL cells. The activity of proteins in the B-cell receptor signalosome and the phosphatidylinositol 3-kinase pathway were altered upon drug exposure. Combined treatment with ibrutinib and venetoclax give promising results in clinical studies and we show that this combination exerted synergistic inhibitory effects on cell signaling and cell viability. Cell viability was monitored by flow cytometry and with independent drug sensitivity screens. Our analyses indicate that the standard dosages of ibrutinib and venetoclax can be lowered without loss of efficacy, potentially reducing drug costs, and toxicities. Observed correlation between signaling and viability indicates that signaling molecules could serve as biomarkers to predict response to therapy. We suggest that phospho flow should be considered as a novel approach for dose and synergy prediction in a precision medicine setting for CLL.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Scarfo L, Ferreri AJ, Ghia P. Chronic lymphocytic leukaemia. Crit Rev Oncol Hematol. 2016;104:169–82.
Lenartova A, Johannesen TB, Tjønnfjord GE. National trends in incidence and survival of chronic lymphocytic leukemia in Norway for 1953-2012: a systematic analysis of population-based data. Cancer Med. 2016;5:3588–95.
Kipps TJ, Stevenson FK, Wu CJ, Croce CM, Packham G, Wierda WG, et al. Chronic lymphocytic leukaemia. Nat Rev Dis Prim. 2017;3:16096.
Zhong Y, Byrd JC, Dubovsky JA. The B-cell receptor pathway: a critical component of healthy and malignant immune biology. Semin Hematol. 2014;51:206–18.
Ten Hacken E, Gounari M, Ghia P, Burger JA. The importance of B cell receptor isotypes and stereotypes in chronic lymphocytic leukemia. Leukemia. 2019;33:287–98.
Arnason JE, Brown JR. Targeting B cell signaling in chronic lymphocytic leukemia. Curr Oncol Rep. 2017;19:61.
Khan M, Siddiqi T. Targeted therapies in CLL: monotherapy versus combination approaches. Curr Hematol Malig Rep. 2018;13:525–33.
Brown JR. The PI3K pathway: clinical inhibition in chronic lymphocytic leukemia. Semin Oncol. 2016;43:260–4.
Falchi L, Baron JM, Orlikowski CA, Ferrajoli A. BCR signaling inhibitors: an overview of toxicities associated with ibrutinib and idelalisib in patients with chronic lymphocytic leukemia. Mediterr J Hematol Infect Dis. 2016;8:e2016011.
Vela CM, McBride A, Jaglowski SM, Andritsos LA. Ibrutinib for treatment of chronic lymphocytic leukemia. Am J Health Syst Pharm. 2016;73:367–75.
Vitale C, Burger JA. Chronic lymphocytic leukemia therapy: new targeted therapies on the way. Expert Opin Pharm. 2016;17:1077–89.
Itchaki G, Brown JR. The potential of venetoclax (ABT-199) in chronic lymphocytic leukemia. Ther Adv Hematol. 2016;7:270–87.
Hillmen P, Rawstron A, Brock K, Munoz Vicente S, Yates F, Bishop R, et al. Ibrutinib plus venetoclax in relapsed/refractory CLL: results of the bloodwise TAP Clarity Study. Blood. 2018;132:182.
Jain N, Keating MJ, Thompson PA, Ferrajoli A, Burger JA, Borthakur G, et al. Ibrutinib and venetoclax for first-line treatment of CLL. N Engl J Med. 2019;380:2095–103.
Chen LS, Bose P, Cruz ND, Jiang Y, Wu Q, Thompson PA, et al. A pilot study of lower doses of ibrutinib in patients with chronic lymphocytic leukemia. Blood. 2018;132:2249–59.
Hermansen JU, Tjønnfjord GE, Munthe LA, Taskén K, Skånland SS. Cryopreservation of primary B cells minimally influences their signaling responses. Sci Rep. 2018;8:17651.
Myhrvold IK, Cremaschi A, Hermansen JU, Tjønnfjord GE, Munthe LA, Taskén K, et al. Single cell profiling of phospho-protein levels in chronic lymphocytic leukemia. Oncotarget. 2018;9:9273–84.
Skånland SS. Phospho flow cytometry with fluorescent cell barcoding for single cell signaling analysis and biomarker discovery. J Vis Exp. 2018;140:e58386.
Bliss C. The toxicity of poisons applied jointly. Ann Appl Biol. 1939;26:585–615.
Berenbaum MC. What is synergy? Pharm Rev. 1989;41:93–141.
Brown JR, Byrd JC, Coutre SE, Benson DM, Flinn IW, Wagner-Johnston ND, et al. Idelalisib, an inhibitor of phosphatidylinositol 3-kinase p110delta, for relapsed/refractory chronic lymphocytic leukemia. Blood. 2014;123:3390–7.
Cheng S, Ma J, Guo A, Lu P, Leonard JP, Coleman M, et al. BTK inhibition targets in vivo CLL proliferation through its effects on B-cell receptor signaling activity. Leukemia. 2014;28:649–57.
Cervantes-Gomez F, Lamothe B, Woyach JA, Wierda WG, Keating MJ, Balakrishnan K, et al. Pharmacological and protein profiling suggests venetoclax (ABT-199) as optimal partner with ibrutinib in chronic lymphocytic leukemia. Clin Cancer Res. 2015;21:3705–15.
Advani RH, Buggy JJ, Sharman JP, Smith SM, Boyd TE, Grant B, et al. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J Clin Oncol. 2013;31:88–94.
Salem AH, Agarwal SK, Dunbar M, Enschede SL, Humerickhouse RA, Wong SL. Pharmacokinetics of venetoclax, a novel BCL-2 inhibitor, in patients with relapsed or refractory chronic lymphocytic leukemia or non-hodgkin lymphoma. J Clin Pharm. 2017;57:484–92.
Herman SE, Mustafa RZ, Gyamfi JA, Pittaluga S, Chang S, Chang B, et al. Ibrutinib inhibits BCR and NF-kappaB signaling and reduces tumor proliferation in tissue-resident cells of patients with CLL. Blood. 2014;123:3286–95.
Zaitseva L, Murray MY, Shafat MS, Lawes MJ, MacEwan DJ, Bowles KM, et al. Ibrutinib inhibits SDF1/CXCR4 mediated migration in AML. Oncotarget. 2014;5:9930–8.
Choudhary GS, Al-Harbi S, Mazumder S, Hill BT, Smith MR, Bodo J, et al. MCL-1 and BCL-xL-dependent resistance to the BCL-2 inhibitor ABT-199 can be overcome by preventing PI3K/AKT/mTOR activation in lymphoid malignancies. Cell Death Dis. 2015;6:e1593.
Pham LV, Huang S, Zhang H, Zhang J, Bell T, Zhou S, et al. Strategic therapeutic targeting to overcome venetoclax resistance in aggressive B-cell lymphomas. Clin Cancer Res. 2018;24:3967–80.
Bodo J, Zhao X, Durkin L, Souers AJ, Philips DC, Smith MR, et al. Acquired resistance to venetoclax (ABT-199) in t(14;18) positive lymphoma cells. Oncotarget. 2016;7:70000–10.
Jayappa KD, Portell CA, Gordon VL, Capaldo BJ, Bekiranov S, Axelrod MJ, et al. Microenvironmental agonists generate de novo phenotypic resistance to combined ibrutinib plus venetoclax in CLL and MCL. Blood Adv. 2017;1:933–46.
Amin NA, Balasubramanian S, Saiya-Cork K, Shedden K, Hu N, Malek SN. Cell-intrinsic determinants of ibrutinib-induced apoptosis in chronic lymphocytic leukemia. Clin Cancer Res. 2017;23:1049–59.
Barr PM, Robak T, Owen C, Tedeschi A, Bairey O, Bartlett NL, et al. Sustained efficacy and detailed clinical follow-up of first-line ibrutinib treatment in older patients with chronic lymphocytic leukemia: extended phase 3 results from RESONATE-2. Haematologica. 2018;103:1502–10.
Fraser G, Cramer P, Demirkan F, Silva RS, Grosicki S, Pristupa A, et al. Updated results from the phase 3 HELIOS study of ibrutinib, bendamustine, and rituximab in relapsed chronic lymphocytic leukemia/small lymphocytic lymphoma. Leukemia. 2019;33:969–80
Thimiri Govinda Raj DB, Cremaschi A, Skånland SS, Gade A, Schjesvold FH, Tjønnfjord GE, et al. In-vitro drug sensitivity screening in chronic lymphocytic leukemia (CLL) primary patient samples identifies drug candidates for precision cancer therapy. Blood. 2018;132:4677.
Acknowledgements
The authors are grateful to all the study participants without whom this study would not have been possible. We thank Martine Schrøder, Marianne Enger, and Gladys M. Tjørhom for expert technical assistance. We are grateful to Alexandra Gade, Johannes Landskron, and Eirin Solberg at the High-Throughput Chemical Biology Screening Platform at Centre for Molecular Medicine Norway, University of Oslo, for assistance with drug sensitivity screens. This work was supported by the Norwegian Cancer Society, the Regional Health Authority for South-Eastern Norway, the Research Council of Norway and Stiftelsen Kristian Gerhard Jebsen (grant number SKGJ-MED-019). SSS was funded by a Career Development Research Fellowship from the Norwegian Cancer Society.
Author information
Authors and Affiliations
Contributions
SSS designed the research. SSS, HB, and JUH performed the experiments and analyzed the data together with AC, LAM, and KT. DBTGR provided methodology. GET and LAM contributed with patient samples and interpreted data. SSS wrote the paper. All authors read and commented on draft versions of the paper and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Skånland, S.S., Cremaschi, A., Bendiksen, H. et al. An in vitro assay for biomarker discovery and dose prediction applied to ibrutinib plus venetoclax treatment of CLL. Leukemia 34, 478–487 (2020). https://doi.org/10.1038/s41375-019-0569-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-019-0569-7
This article is cited by
-
A tumor microenvironment model of chronic lymphocytic leukemia enables drug sensitivity testing to guide precision medicine
Cell Death Discovery (2023)
-
Determining drug dose in the era of targeted therapies: playing it (un)safe?
Blood Cancer Journal (2022)
-
PI3K inhibitors are finally coming of age
Nature Reviews Drug Discovery (2021)