Abstract
Background
Biomonitoring data and determinants of urinary dialkylphosphate (DAP) metabolites, markers of organophosphate pesticides, in racially diverse, non-occupationally exposed populations are scarce.
Objective
This study evaluated urinary concentrations and potential determinants of DAP metabolites of organophosphate pesticides in a multi-site, multi-racial/ethnic cohort of women aged 45–56 years, the Study of Women’s Health Across the Nation Multi-Pollutant Study (SWAN-MPS).
Methods
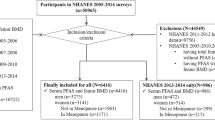
We analyzed 963 urine samples collected in 1999–2000, the baseline of SWAN-MPS for longitudinal studies, and quantified DAP metabolites, including dimethyl alkylphosphates (DMAPs): dimethylphosphate (DMP), dimethylthiophosphate (DMTP), dimethyldithiophosphate (DMDTP); and diethyl alkylphosphates (DEAPs): diethylphosphate (DEP), diethylthiophosphate (DETP), diethyldithiophosphate (DEDTP), using gas chromatography and triple quadrupole mass spectroscopy. Adjusted least squared geometric means (LSGMs) and 95% confidence intervals (CIs) were computed to compare DAP concentrations by socio-demographic, behavioral and dietary factors.
Results
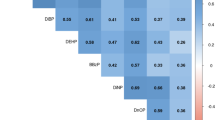
The geometric means (geometric standard deviations) of total DAPs, DMAPs, and DEAPs were 141 (2.63) nmol/L, 102 (2.99) nmol/L, and 26.8 (2.46) nmol/L, respectively. Body mass index (BMI) was inversely associated with DMAPs and DEAPs: LSGM (95% CI) = 68.8 (55.7–84.9) and 21.0 (17.7–25.0) nmol/L for women with obesity vs. 102 (84.7–123) and 30.1 (25.7–35.1) nmol/L for women with normal/underweight, respectively. Fruit consumption was positively (74.9 (62.1–90.2) for less than 5–6 servings/week vs. 105 (84.8–130) nmol/L for 1 serving/day and more) whereas meat consumption was inversely associated with DMAPs (110 (95.0–128) for seldom vs. 82.3 (59.5–114) nmol/L for often consumption). Fresh apple consumption appears to be attributed to the DMAP differences. Alcohol consumption was positively associated with DEAPs (27.5 (23.1–32.7) for 2 drinks/week and more vs. 23.0 (20.0–26.6) nmol/L for less than 1 drink/month). Black women had higher concentrations of DEAPs compared with White women (27.3 (21.2–35.2) vs. 23.2 (20.2–26.7) nmol/L).
Impact Statement
Organophosphate pesticides (OPs) are synthetic chemicals and currently the most widely used type of insecticides. We examined multi-site, multi-ethnic cohort of midlife women in the U.S. that offers a unique opportunity to evaluate major determinants of OP exposure. We improved OP metabolite detection rates and obtained accurate concentrations using an improved analytical technique. Our findings suggest that consumptions of fruit, meat and alcohol are important determinants of OP exposure for midlife women. Higher concentrations of diethyl OP metabolites in Black women compared to White women, even after accounting for dietary intake, suggests additional, but unknown racial-ethnic differences that affect exposure.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
SWAN provides access to public use datasets that include data from SWAN screening, the baseline visit and follow-up visits (https://agingresearchbiobank.nia.nih.gov/). To preserve participant confidentiality, some, but not all, of the data used for this manuscript are contained in the public use datasets. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided. A link to the public use datasets is also located on the SWAN web site: http://www.swanstudy.org/swan-research/data-access/. Investigators who require assistance accessing the public use dataset may contact the SWAN Coordinating Center at the following email address: swanaccess@edc.pitt.edu.
References
Kavvalakis MP, Tsatsakis AM. The atlas of dialkylphosphates; assessment of cumulative human organophosphorus pesticides’ exposure. Forensic Sci Int. 2012;218:111–22.
Earthjustice. Where are organophosphate pesticides used? 2021.
Bidleman TF, Leone AD. Soil-air exchange of organochlorine pesticides in the Southern United States. Environ Pollut. 2004;128:49–57.
Shen L, Wania F, Lei YD, Teixeira C, Muir DCG, Bidleman TF. Atmospheric distribution and long-range transport behavior of organochlorine pesticides in North America. Environ Sci Technol. 2005;39:409–20.
WHO (World Health Organization). Pesticide. 2018.
US EPA (United States Environmental Protection Agency). Pesticides. 2022.
Jayatilaka NK, Restrepo P, Davis Z, Vidal M, Calafat AM, Ospina M. Quantification of 16 urinary biomarkers of exposure to flame retardants, plasticizers, and organophosphate insecticides for biomonitoring studies. Chemosphere. 2019;235:481–91.
Dell’Omo G, Bryenton R, Shore RF. Effects of exposure to an organophosphate pesticide on behavior and acetylcholinesterase activity in the common shrew, Sorex araneus. Environ Toxicol Chem. 1997;16:272–6.
dos Santos AA, Naime AA, de Oliveira J, Colle D, dos Santos DB, Hort MA, et al. Long-term and low-dose malathion exposure causes cognitive impairment in adult mice: evidence of hippocampal mitochondrial dysfunction, astrogliosis and apoptotic events. Arch Toxicol. 2016;90:647–60.
Terry AV. Functional consequences of repeated organophosphate exposure: potential non-cholinergic mechanisms. Pharm Ther. 2012;134:355–65.
Meyer-Baron M, Knapp G, Schäper M, van Thriel C. Meta-analysis on occupational exposure to pesticides - Neurobehavioral impact and dose-response relationships. Environ Res. 2015;136:234–45.
Savy CY, Fitchett AE, Blain PG, Morris CM, Judge SJ. Gene expression analysis reveals chronic low level exposure to the pesticide diazinon affects psychological disorders gene sets in the adult rat. Toxicology. 2018;393:90–101.
Androutsopoulos VP, Hernandez AF, Liesivuori J, Tsatsakis AM. A mechanistic overview of health associated effects of low levels of organochlorine and organophosphorous pesticides. Toxicology. 2013;307:89–94.
De Angelis S, Tassinari R, Maranghi F, Eusepi A, Di Virgilio A, Chiarotti F, et al. Developmental exposure to chlorpyrifos induces alterations in thyroid and thyroid hormone levels without other toxicity signs in CD-1 mice. Toxicol Sci. 2009;108:311–9.
Haviland JA, Butz DE, Porter WP. Long-term sex selective hormonal and behavior alterations in mice exposed to low doses of chlorpyrifos in utero. Reprod Toxicol. 2010;29:74–79.
Slotkin TA, Seidler FJ. Oxidative stress from diverse developmental neurotoxicants: Antioxidants protect against lipid peroxidation without preventing cell loss. Neurotoxicol Teratol. 2010;32:124–31.
Zafiropoulos A, Tsarouhas K, Tsitsimpikou C, Fragkiadaki P, Germanakis I, Tsardi M, et al. Cardiotoxicity in rabbits after a low-level exposure to diazinon, propoxur, and chlorpyrifos. Hum Exp Toxicol. 2014;33:1241–52.
Slotkin TA, Bodwell BE, Levin ED, Seidler FJ. Neonatal exposure to low doses of diazinon: long-term effects of neural cell development and acetylcholine systems. Environ Health Perspect. 2008;116:340–8.
Katsikantami I, Colosio C, Alegakis A, Tzatzarakis MN, Vakonaki E, Rizos AK, et al. Estimation of daily intake and risk assessment of organophosphorus pesticides based on biomonitoring data – The internal exposure approach. Food Chem Toxicol. 2019;123:57–71.
Eskenazi B, Bradman A, Castorina R. Exposures of children to organophosphate pesticides and their potential adverse health effects. Environ Health Perspect. 1999;107:409–19.
Bräuner EV, Raaschou-Nielsen O, Gaudreau E, Leblanc A, Tjønneland A, Overvad K, et al. Predictors of adipose tissue concentrations of organochlorine pesticides in a general Danish population. J Expo Sci Environ Epidemiol. 2012;22:52–59.
Poet TS, Wu H, Kousba AA, Timchalk C. In vitro rat hepatic and intestinal metabolism of the organophosphate pesticides chlorpyrifos and diazinon. Toxicol Sci. 2003;72:193–200.
Ganie SY, Javaid D, Hajam YA, Reshi MS. Mechanisms and treatment strategies of organophosphate pesticide induced neurotoxicity in humans: A critical appraisal. Toxicology. 2022;472:153181.
Roberts EM, English PB, Grether JK, Windham GC, Somberg L, Wolff C. Maternal residence near agricultural pesticide applications and autism spectrum disorders among children in the California Central Valley. Environ Health Perspect. 2007;115:1482–9.
Baer, KN. Malathion. In: Encyclopedia of Toxicology. 2nd ed. 2005.
WHO (World Health Organization). International Programme on Chemical Safety (IPCS)/INCHEM. Organophosphorus Pesticides Environ Heal Criteria. 1986;63:1986.
CDC (Centers for Disease Control and Prevention). Organophosphorus Insecticides: Dialkyl Phosphate Metabolites. 2017.
Minton NA, Murray VS. A review of organophosphate poisoning. Med Toxicol Advers Drug Exp. 1988;3:350–75.
Barr DB, Landsittel D, Nishioka M, Thomas K, Curwin B, Raymer J, et al. A survey of laboratory and statistical issues related to farmworker exposure studies. Environ Health Perspect. 2006;114:961–8.
Barr DB, Bravo R, Weerasekera G, Caltabiano LM, Whitehead RD, Olsson AO, et al. Concentrations of dialkyl phosphate metabolites of organophosphorus pesticides in the US population. Environ Health Perspect. 2004;112:186–200.
Sokoloff K, Fraser W, Arbuckle TE, Fisher M, Gaudreau E, LeBlanc A, et al. Determinants of urinary concentrations of dialkyl phosphates among pregnant women in Canada - Results from the MIREC study. Environ Int. 2016;94:133–40.
van den Dries MA, Pronk A, Guxens M, Spaan S, Voortman T, Jaddoe VW, et al. Determinants of organophosphate pesticide exposure in pregnant women: A population-based cohort study in the Netherlands. Int J Hyg Environ Health. 2018;221:489–501.
Yolton K, Xu Y, Sucharew H, Succop P, Altaye M, Popelar A, et al. Impact of low-level gestational exposure to organophosphate pesticides on neurobehavior in early infancy: a prospective study. Environ Heal. 2013;12:79.
Llop S, Murcia M, Iñiguez C, Roca M, González L, Yusà V, et al. Distributions and determinants of urinary biomarkers of organophosphate pesticide exposure in a prospective Spanish birth cohort study. Environ Heal A Glob Access Sci Source. 2017;16:3–5.
Lewis RC, Cantonwine DE, Anzalota Del Toro LV, Calafat AM, Valentin-Blasini L, Davis MD, et al. Distribution and determinants of urinary biomarkers of exposure to organophosphate insecticides in Puerto Rican pregnant women. Sci Total Environ. 2015;512–513:337–44.
Bravo R, Driskell WJ, Whitehead RD, Needham LL, Barr DB. Quantitation of dialkyl phosphate metabolites of organophosphate pesticides in human urine using GC-MS-MS with isotopic internal standards. J Anal Toxicol. 2002;26:245–52.
US EPA (United States Environmental Protection Agency). Quality Assurance (QA)-Quality Control (QC). 2022.
CDC (Centers for Disease Control and Prevention). National Health and Nutrition Examination Survey 2001-2002. 2013.
Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data-based approach to diet questionnaire design and testing. Am J Epidemiol. 1986;124:453–69.
Thomatou AA, Zacharias I, Hela D, Konstantinou I. Determination and risk assessment of pesticide residues in lake Amvrakia (W. Greece) after agricultural land use changes in the lake’s drainage basin. Int J Environ Anal Chem. 2013;93:780–99.
Frank R, Braun HE, Chapman N, Burchat C. Degradation of parent compounds of nine organophosphorus insecticides in Ontario surface and ground waters under controlled conditions. Bull Environ Contam Toxicol. 1991;47:374–80.
US EPA (United States Environmental Protection Agency). Regional Guidance on Handling Chemical Concentration Data Near the Detection Limit in Risk Assessments. 1991.
Lee G, Kim S, Park H, Lee J, Lee JP, Kho Y, et al. Variability of urinary creatinine, specific gravity, and osmolality over the course of pregnancy: Implications in exposure assessment among pregnant women. Environ Res. 2021;198:110473.
Wasserstein RL, Lazar NA. The ASA’s Statement on p-Values: Context, Process, and Purpose. Am Stat. 2016;70:129–33.
Bates N, Campbell A Chapter 5: Organophosphate Insecticides. Handb. Poisoning Dogs Cats. 2008;199–204.
Alwis GKH, De, Needham LL, Barr DB. Determination of dialkyl phosphate metabolites of organophosphorus pesticides in human urine by automated solid-phase extraction, derivatization, and gas chromatography-mass spectrometry. Chem. J Anal Toxicol. 2008;32:721–7.
NPIC (National Pesticide Information Center). Malathion. 2010.
Nolan R, Rick D, Freshour N, Saunders J. Chlorpyrifos: pharmacokinetics in Human Volunteers. Toxicol Appl Pharm. 1984;73:8–15.
Ojha A, Yaduvanshi SK, Srivastava N. Effect of combined exposure of commonly used organophosphate pesticides on lipid peroxidation and antioxidant enzymes in rat tissues. Pestic Biochem Physiol. 2011;99:148–56.
CDC (Centers for Disease Control and Prevention). National Report on Human Exposure to Environmental Chemicals. 2018.
Ye X, Pierik FH, Hauser R, Duty S, Angerer J, Park MM, et al. Urinary metabolite concentrations of organophosphorous pesticides, bisphenol A, and phthalates among pregnant women in Rotterdam, the Netherlands: The Generation R study. Environ Res. 2008;108:260–7.
Kimmons J, Gillespie C, Seymour J, Serdula M, Blanck HM. Fruit and Vegetable Intake Among Adolescents and Adults in the United States: Percentage Meeting Individualized Recommendations. Medscape J Med. 2009;11:26.
WHO (World Health Organization). Measuring intake of fruit and vegetables. 2005.
OECD (Organization for Economic Cooperation and Development). OECD Environmental Performance Reviews: The Netherlands 2015. 2015.
EU (European Union). Monitoring of pesticide residues in products of plant origin in European union, Norway, Iceland and Liechtenstein, 2006 Report. 2008.
CBS (Centraal Bureau voor de Statistiek). Statistics Netherlands. 2017.
EU (European Union). European Union: New Rules Propose to Halve Pesticide Use and Risk in the EU. 2022.
PE (Pan-Europe). Banned and Hazardous Pesticides in European Food. 2020.
Witczak A, Pohoryło A, Abdel-Gawad H, Cybulski J. Residues of some organophosphorus pesticides on and in fruits and vegetables available in Poland, an assessment based on the European union regulations and health assessment for human populations. Phosphorus Sulfur Silicon Relat Elem. 2018;193:711–20.
Cabras P, Angioni A. Pesticide residues in grapes, wine, and their processing products. J Agric Food Chem. 2000;48:967–73.
True Sport. Is organic worth it: 7 things to know about buying organic. 2021.
Peña JE, Mohyuddin AI, Wysoki M. A review of the pest management situation in mango agroecosystems. Phytoparasitica. 1998;26:129–48.
Edwards CA. Factors that affect the persistence of pesticides in plants and soils. Pestic Chem. 1975;42:39–56.
Wang Y, Beydoun MA. Meat consumption is associated with obesity and central obesity among US adults. Int J Obes. 2009;33:621–8.
Brill MJE, Diepstraten J, Van Rongen A, Van Kralingen S, Van Den Anker JN, Knibbe CAJ. Impact of obesity on drug metabolism and elimination in adults and children. Clin Pharmacokinet. 2012;51:277–304.
Acknowledgements
The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants U01NR004061; U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495, and U19AG063720). The study was also supported by the SWAN Repository (U01AG017719). This publication was supported in part by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number UL1 RR024131. This study was also supported by grants from the National Institute of Environmental Health Sciences (NIEHS) R01-ES026578, R01-ES026964, R01-ES035087 and P30-ES017885, and by the Center for Disease Control and Prevention (CDC)/National Institute for Occupational Safety and Health (NIOSH) grant T42-OH008455. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH. Clinical Centers: University of Michigan, Ann Arbor—Carrie Karvonen-Gutierrez, PI 2021 – present, Siobán Harlow, PI 2011–2021, MaryFran Sowers, PI 1994–2011; Massachusetts General Hospital, Boston, MA—Sherri‐Ann Burnett‐Bowie, PI 2020—Present; Joel Finkelstein, PI 1999–2020; Robert Neer, PI 1994–1999; Rush University, Rush University Medical Center, Chicago, IL—Imke Janssen, PI 2020—Present; Howard Kravitz, PI 2009–2020; Lynda Powell, PI 1994–2009; University of California, Davis/Kaiser—Elaine Waetjen and Monique Hedderson, PIs 2020—Present; Ellen Gold, PI 1994–2020; University of California, Los Angeles—Arun Karlamangla, PI 2020—Present; Gail Greendale, PI 1994–2020; Albert Einstein College of Medicine, Bronx, NY—Carol Derby, PI 2011—present, Rachel Wildman, PI 2010–2011; Nanette Santoro, PI 2004–2010; University of Medicine and Dentistry—New Jersey Medical School, Newark—Gerson Weiss, PI 1994–2004; and the University of Pittsburgh, Pittsburgh, PA – Rebecca Thurston, PI 2020—Present; Karen Matthews, PI 1994–2020. NIH Program Office: National Institute on Aging, Bethesda, MD—Rosaly Correa-de-Araujo 2020—present; Chhanda Dutta 2016–2020; Winifred Rossi 2012–2016; Sherry Sherman 1994–2012; Marcia Ory 1994–2001; National Institute of Nursing Research, Bethesda, MD—Program Officers. Central Laboratory: University of Michigan, Ann Arbor—Daniel McConnell (Central Ligand Assay Satellite Services). NIA Biorepository: Rosaly Correa-de-Araujo 2019—Present; SWAN Repository: University of Michigan, Ann Arbor—Siobán Harlow 2013–2018; Dan McConnell 2011–2013; MaryFran Sowers 2000–2011. Coordinating Center: University of Pittsburgh, Pittsburgh, PA—Maria Mori Brooks, PI 2012—present; Kim Sutton-Tyrrell, PI 2001–2012; New England Research Institutes, Watertown, MA—Sonja McKinlay, PI 1995–2001. Steering Committee: Susan Johnson, Current Chair. Chris Gallagher, Former Chair. We thank the study staff at each site and all the women who participated in SWAN. We also thank Drs. Habyeong Kang and Ning Ding for their help in data analysis.
Author information
Authors and Affiliations
Contributions
Sung-Hee Seo was responsible for the measurement of urinary DAPs, data analysis, interpretation of results, and writing—original draft, and revisions. Stuart Batterman oversaw DAPs method development and measurement analysis, laboratory administration, and contributed to results interpretation and manuscript revisions. Carrie Karvonen-Gutierrez contributed to funding acquisition, project administration, and manuscript revisions. Sung Kyun Park is the guarantor of this work and had full access to all the data in the study and takes responsibility for the contents of the manuscript. Sung Kyun Park was responsible for funding acquisition, study design protocols, oversight of statistical analysis, interpretation of results, project administration, and writing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Seo, SH., Batterman, S., Karvonen-Gutierrez, C.A. et al. Determinants of urinary dialkyl phosphate metabolites in midlife women: the Study of Women’s Health Across the Nation Multi-Pollutant Study (SWAN-MPS). J Expo Sci Environ Epidemiol (2024). https://doi.org/10.1038/s41370-024-00672-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41370-024-00672-z