Abstract
Background/objectives
Proinflammatory cytokines are increased in obese adipose tissue, including inflammasome key masters. Conversely, IL-18 protects against obesity and metabolic dysfunction. We focused on the IL-18 effect in controlling adipose tissue remodeling and metabolism.
Materials/subjects and methods
We used C57BL/6 wild-type (WT) and interleukine-18 deficient (IL-18−/−) male mice fed a chow diet and samples from bariatric surgery patients.
Results
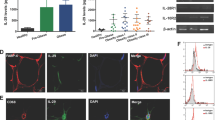
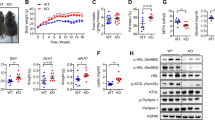
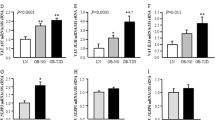
IL-18−/− mice showed increased adiposity and proinflammatory cytokine levels in adipose tissue, leading to glucose intolerance. IL-18 was widely secreted by stromal vascular fraction but not adipocytes from mice’s fatty tissue. Chimeric model experiments indicated that IL-18 controls adipose tissue expansion through its presence in tissues other than bone marrow. However, IL-18 maintains glucose homeostasis when present in bone marrow cells. In humans with obesity, IL-18 expression in omental tissue was not correlated with BMI or body fat mass but negatively correlated with IRS1, GLUT-4, adiponectin, and PPARy expression. Also, the IL-18RAP receptor was negatively correlated with IL-18 expression.
Conclusions
IL-18 signaling may control adipose tissue expansion and glucose metabolism, as its absence leads to spontaneous obesity and glucose intolerance in mice. We suggest that resistance to IL-18 signaling may be linked with worse glucose metabolism in humans with obesity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–45.
Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Invest. 2011;6:2094–101.
Wood IS, Wang B, Jenkins JR, Trayhurn P. The pro-inflammatory cytokine IL-18 is expressed in human adipose tissue and strongly upregulated by TNFalpha in human adipocytes. Biochem Biophys Res Commun. 2005;337:422–9.
Akdis M, Burgler S, Crameri R, Eiwegger T, Fujita H, Gomez E, et al. Interleukins, from 1 to 37, and interferon-γ: receptors, functions, and roles in diseases. J Allergy Clin Immunol. 2011;127:701–21.
Point W, Dinarello CA. IL-18: A T H 1 -inducing, proinflammatory cytokine and new member of the IL-1 family. J Allergy Clin Immunol. 1999;103:11–24.
Dao T, Ohashi K, Kayano T, Kurimoto M, Okamura H. Interferon-gamma-inducing factor, a novel cytokine, enhances Fas ligand-mediated cytotoxicity of murine T helper 1 cells. Cell Immunol. 1996;173:230–5.
Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 is a unique cytokine that stimulates both Th1 and Th2 responses depending on its cytokine milieu. Cytokine Growth Factor Rev. 2001;12:53–72.
Puren AJ, Fantuzzi G, Gu Y, Su MS, Dinarello CA. Interleukin-18 (IFN ␥ -inducing Factor) Induces IL-8 and IL-1  via TNF ␣ Production. J Clin Invest. 1998;101:711–21.
Netea MG, Kullberg BJ, Verschueren I, Van Der Meer JW. Interleukin‐18 induces production of proinflammatory cytokines in mice: no intermediate role for the cytokines of the tumor necrosis factor family and interleukin‐1β. Eur J Immunol. 2000;30:3057–60.
Kashiwamura S, Ueda H, Okamura H. Roles of interleukin-18 in tissue destruction and compensatory reactions. J Immunother. 2002;25:4–11.
Netea MG, Joosten LA, Lewis E, Jensen DR, Voshol PJ, Kullberg BJ, et al. Deficiency of interleukin-18 in mice leads to hyperphagia, obesity and insulin resistance. Nat Med. 2006;12:650–6.
Lindegaard B, Matthews VB, Brandt C, Hojman P, Syberg S, Rudnicka C, et al. Interleukin-18 activates skeletal muscle AMPK and reduces weight gain and insulin resistance in mice. Diabetes. 2013;62:1–42.
Akira S. The role of IL-18 in innate immunity. Curr Opin Immunol. 2000;12:59–63.
Esposito K. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation. 2002;106:2067–72.
Olusi SO, Al-Awadhi A, Abraham M. Relations of serum interleukin 18 levels to serum lipid and glucose concentrations in an apparently healthy adult population. Horm Res. 2003;60:29–33.
Zilverschoon GRC, Tack CJ, Joosten LAB, Kullberg BJ, Van Der Meer JWM, Netea MG. Interleukin-18 resistance in patients with obesity and type 2 diabetes mellitus. Int J Obes. 2008;32:1407–14. https://doi.org/10.1038/ijo.2008.109.
Rodbell M. Metabolism of isolated fat cells. I. Effects of hormones on glucose metabolism and lipolysis. J Biol Chem. 1964;239:375–80.
de Assis-Ferreira, Saldanha-Gama R, de Brito NM, Renovato-Martins M, Simões RL, Barja-Fidalgo C, Vargas da Silva S. Obesity enhances the recruitment of mesenchymal stem cells to visceral adipose tissue. J Mol Endocrinol. 2021;67:15–26. https://doi.org/10.1530/JME-20-0229.
da Silva SV, Renovato-Martins M, Ribeiro-Pereira C, Citelli M, Barja-Fidalgo C. Obesity modifies bone marrow microenvironment and directs bone marrow mesenchymal cells to adipogenesis. Obesity. 2016;24. https://doi.org/10.1002/oby.21660.
Emont MP, Jacobs C, Essene AL, Pant D, Tenen D, Colleluori G, et al. A single-cell atlas of human and mouse white adipose tissue. Nature. 2022;603:926–933.
Castor MGM, Rezende B, Resende CB, Alessandri AL, Fagundes CT, Sousa LP, et al. The CCL3/Macrophage Inflammatory Protein-1α–Binding Protein Evasin-1 Protects from Graft-versus-Host Disease but Does Not Modify Graft-versus-Leukemia in Mice. The Journal of Immunology 2010;184. https://doi.org/10.4049/jimmunol.0902614.
Divoux A, Tordjman J, Lacasa D, Veyrie N, Hugol D, Aissat A, et al. Fibrosis in human adipose tissue: Composition, distribution, and link with lipid metabolism and fat mass loss. Diabetes. 2010;59. https://doi.org/10.2337/db10-0585.
Aron-Wisnewsky J, Julia Z, Poitou C, Bouillot JL, Basdevant A, Chapman MJ et al. Effect of bariatric surgery-induced weight loss on SR-BI-, ABCG1-, and ABCA1-mediated cellular cholesterol efflux in obese women. J Clin Endocrinol Metab. 2011;96. https://doi.org/10.1210/jc.2010-2378.
Zorrilla EP, Sanchez-Alavez M, Sugama S, Brennan M, Fernandez R, Bartfai T, et al. Interleukin-18 controls energy homeostasis by suppressing appetite and feed efficiency. Proc Natl Acad Sci USA. 2007;104. https://doi.org/10.1073/pnas.0611523104.
Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334. https://doi.org/10.1056/nejm199602013340503.
Frederich RC, Hamann A, Anderson S, Löllmann B, Lowell BB, Flier JS. Leptin levels reflect body lipid content in mice: Evidence for diet-induced resistance to leptin action. Nat Med. 1995;1. https://doi.org/10.1038/nm1295-1311.
Zhang Y, Guo KY, Diaz PA, Heo M, Leibel RL. Determinants of leptin gene expression in fat depots of lean mice. Am J Physiol Regul Integr Comp Physiol. 2002;282. https://doi.org/10.1152/ajpregu.00392.2001.
Reaven GM. Pathophysiology of insulin resistance in human disease. Physiol Rev. 1995;75. https://doi.org/10.1152/physrev.1995.75.3.473.
Wang RH, Kim HS, Xiao C, Xu X, Gavrilova O, Deng CX. Hepatic Sirt1 deficiency in mice impairs mTorc2/Akt signaling and results in hyperglycemia, oxidative damage, and insulin resistance. J Clin Investig. 2011;121. https://doi.org/10.1172/JCI46243.
Gross DN, Van Den Heuvel APJ, Birnbaum MJ. The role of FoxO in the regulation of metabolism. Oncogene. 2008;27. https://doi.org/10.1038/onc.2008.25.
Wang H, Capell W, Yoon JH, Faubel S, Eckel RH. Obesity development in caspase-1-deficient mice. Int J Obes 2014;38. https://doi.org/10.1038/ijo.2013.59.
Kimura H, Karasawa T, Usui F, Kawashima A, Endo Y, Kobayashi M, et al. Caspase-1 deficiency promotes high-fat diet-induced adipose tissue inflammation and the development of obesity. Am J Physiol Endocrinol Metab. 2016;311. https://doi.org/10.1152/ajpendo.00174.2016.
Stienstra R, Joosten LAB, Koenen T, Van Tits B, Van Diepen JA, Van Den Berg SAA, et al. The inflammasome-mediated caspase-1 activation controls adipocyte differentiation and insulin sensitivity. Cell Metab. 2010;12. https://doi.org/10.1016/j.cmet.2010.11.011.
Kotas ME, Jurczak MJ, Annicelli C, Gillum MP, Cline GW, Shulman GI, et al. Role of caspase-1 in regulation of triglyceride metabolism. Proc Natl Acad Sci USA. 2013;110. https://doi.org/10.1073/pnas.1301996110.
Zhang X, Luo S, Wang M, Cao Q, Zhang Z, Huang Q, et al. Differential IL18 signaling via IL18 receptor and Na-Cl co-transporter discriminating thermogenesis and glucose metabolism regulation. Nat Commun. 2022;13. https://doi.org/10.1038/s41467-022-35256-8.
Dinarello CA, Novick D, Kim S, Kaplanski G. Interleukin-18 and IL-18 binding protein. Front Immunol. 2013;4. https://doi.org/10.3389/fimmu.2013.00289.
Murphy AJ, Kraakman MJ, Kammoun HL, Dragoljevic D, Lee MKS, Lawlor KE, et al. IL-18 production from the NLRP1 inflammasome prevents obesity and metabolic syndrome. Cell Metab. 2016;23. https://doi.org/10.1016/j.cmet.2015.09.024.
Ahmad R, Thomas R, Kochumon S, Sindhu S. Increased adipose tissue expression of IL-18R and its Ligand IL-18 associates with inflammation and insulin resistance in obesity. Immun Inflamm Dis. 2017;5. https://doi.org/10.1002/iid3.170.
Hung J, McQuillan BM, Chapman CML, Thompson PL, Beilby JP. Elevated interleukin-18 levels are associated with the metabolic syndrome independent of obesity and insulin resistance. Arterioscler Thromb Vasc Biol. 2005;25. https://doi.org/10.1161/01.ATV.0000163843.70369.12.
Zhuang H, Han J, Cheng L, Liu SL. A positive causal influence of IL-18 levels on the risk of T2DM: a Mendelian randomization study. Front Genet. 2019;10. https://doi.org/10.3389/fgene.2019.00295.
Gateva A, Kamenov Z, Karamfilova V, Assyov Y, Velikova T, El-Darawish Y, et al. Higher levels of IL-18 in patients with prediabetes compared to obese normoglycaemic controls. Arch Physiol Biochem. 2020;126. https://doi.org/10.1080/13813455.2018.1555667.
Oliveira MS, Rheinheimer J, Moehlecke M, Rodrigues M, Assmann TS, Leitão CB, et al. UCP2, IL18, and miR-133a-3p are dysregulated in subcutaneous adipose tissue of patients with obesity. Mol Cell Endocrinol. 2020;509. https://doi.org/10.1016/j.mce.2020.110805.
Trøseid M, Seljeflot I, Arnesen H. The role of interleukin-18 in the metabolic syndrome. Cardiovasc Diabetol. 2010;9. https://doi.org/10.1186/1475-2840-9-11.
Giorgino F, Leonardini A, Laviola L, Perrini S, Natalicchio A. Cross-talk between PPARγ and insulin signaling and modulation of insulin sensitivity. PPAR Res. 2009. https://doi.org/10.1155/2009/818945.
Young MT, Phelan MJ, Nguyen NT. A Decade Analysis of Trends and Outcomes of Male vs Female Patients Who Underwent Bariatric Surgery. J Am Coll Surg. 2016;222:226–231.
Acknowledgements
This work was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Comité français d’Evaluation de la Coopération Universitaire et Scientifique avec le Brésil (COFECUB) grants no 88887.130206/2017-01 and 88887.879203/2023-01. We thank Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), Pró-Reitoria de Pesquisa (PRPq) from UFMG, National Council for Scientific and Technological Development (CNPq/Brazil) by financial support, and National Agency of Research (e.g., Captor program) and the French Foundation for Medical Research, FRM. The clinical investigation was performed at the Human Nutrition Research Center (CRNH Ile de France), Pitié-Salpêtrière Hospital. We also thank Florence Marchelli, APHP, for data management and Professor Leda Vieira for providing the IL-18 knockout mice.
Author information
Authors and Affiliations
Contributions
JPL, MCO, GM, and AVMF: conceptualization and study design; JPL, MCO, ALMS, LTPY, KAC, AAF, and SD: Investigation; SVS, ELG, VP, MMT, GM, KC, AVMF: funding acquisition and resources; JPL, MCO, ALMS, SVS, ELG, GM, KC, and AVMF: writing—original draft, review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lana, J.P., de Oliveira, M.C., Silveira, A.L.M. et al. Role of IL-18 in adipose tissue remodeling and metabolic dysfunction. Int J Obes (2024). https://doi.org/10.1038/s41366-024-01507-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41366-024-01507-5