Abstract
Aims
Over the past several decades, many antiobesity drugs have been withdrawn from the market due to unanticipated adverse events, often involving cardiotoxicity. This study aimed to evaluate the presence of cardiovascular safety signals with currently marketed antiobesity drugs.
Methods
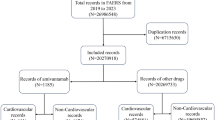
We used the US Food and Drug Administration Adverse Event Reporting System (FAERS) database and retrieved data from January 2013 through December 2018. We performed disproportionality analyses to detect cardiovascular safety signals with three antiobesity drugs recently approved for marketing: lorcaserin, naltrexone–bupropion, phentermine, and phentermine–topiramate. Three main cardiovascular outcomes were evaluated: valvular disorders, and pulmonary hypertension (PH) and other cardiovascular events (myocardial infarction, stroke, cardiovascular death, cardiac failure, and arrhythmia).
Results
During the evaluated period, a total of 6,787,840 adverse event reports were submitted to FAERS. Of these, 2687 involved lorcaserin, 3960 involved phentermine/phentermine–topiramate, and 2873 involved naltrexone–bupropion. Lorcaserin was associated with a significantly greater proportion of reports of valvular disorders (ROR = 4.39; 95% CI 2.72–5.07). None of the antiobesity drugs were associated with a safety signal for valvulopathy, PH, or other cardiovascular events.
Conclusions
Our analyses revealed a signal for valvular disorders with lorcaserin and did not detect a safety signal for other cardiovascular events with recently approved antiobesity drugs. Further research is needed to explore and validate this signal.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
World Health Organization. Overweight and obesity. 2017. http://www.who.int/gho/ncd/risk_factors/overweight_obesity/obesity_adults/en/. Accessed 29 May 2018.
Fleming JW, McClendon KS, Riche DM. New obesity agents: lorcaserin and phentermine/topiramate. Ann Pharmacother. 2013;47:1007–16.
Centers for Disease Control and Prevention. Adult obesity facts. 2018. https://www.cdc.gov/obesity/data/adult.html. Accessed 14 Sep 2018.
Krentz AJ, Fujioka K, Hompesch M. Evolution of pharmacological obesity treatments: focus on adverse side-effect profiles. Diabetes Obes Metab. 2016;18:558–70.
Dietz WH, Baur LA, Hall K, Puhl RM, Taveras EM, Uauy R, et al. Management of obesity: improvement of health-care training and systems for prevention and care. Lancet. 2015;385:2521–33. https://doi.org/10.1016/S0140-6736(14)61748-7.
Onakpoya IJ, Heneghan CJ, Aronson JK. Post-marketing withdrawal of anti-obesity medicinal products because of adverse drug reactions: a systematic review. BMC Med. 2016;14:1–11. https://doi.org/10.1186/s12916-016-0735-y.
Levy RJ. Serotonin transporter mechanisms and cardiac disease. Circulation. 2006;113:2–4.
Jick Hershel, Vasilakis C, Weinrauch LA, Meier CR, Jick SS, Derby LE. A population-based study of appetite-suppressant drugs and the risk of cardiac-valve regurgitation. N Engl J Med. 1998;339:719–24.
Rothman RB, Baumann MH, Savage JE, Rauser L, McBride A, Hufeisen SJ, et al. Evidence for possible involvement of 5-HT(2B) receptors in the cardiac valvulopathy associated with fenfluramine and other serotonergic medications. Circulation. 2000;102:2836–41. http://www.ncbi.nlm.nih.gov/pubmed/11104741.
Michelakis Evangelos D, Weir EK. Anorectic drugs and pulmonary hypertension from the bedside to the bench. Am J Med Sci. 2001;321:292–9. https://www.sciencedirect.com/science/article/pii/S0002962915346772?via%3Dihub.
Watts SW, Davis RP. 5-Hydroxtryptamine receptors in systemic hypertension: an arterial focus. Cardiovasc Ther. 2012;29:54–67. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2943543/pdf/nihms209317.pdf.
U.S. Food and Drug Administration (FDA). FDA Drug Safety Communication: FDA recommends against the continued use of Meridia (sibutramine). U.S. Food and Drug Administration; 2010. FDA announcement available at https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fdarecommends-against-continued-use-meridia-sibutramine. Accessed 16 Feb 2020.
James WPT, Caterson ID, Coutinho W, Finer N, Van Gaal LF, Maggioni AP. et al. Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. N Engl J Med. 2010;363:905–17. https://doi.org/10.1056/NEJMoa1003114.
Caterson ID, Finer N, Coutinho W, Van Gaal LF, Maggioni AP, Torp-Pedersen C, et al. Maintained intentional weight loss reduces cardiovascular outcomes: results from the Sibutramine Cardiovascular OUTcomes (SCOUT) trial. Diabetes Obes Metab. 2012;14:523–30. https://doi.org/10.1111/j.1463-1326.2011.01554.x.
Haslam D. Weight management in obesity—past and present. Int J Clin Pract. 2016;70:206–17.
Patel DK, Stanford FC. Safety and tolerability of new-generation anti-obesity medications: a narrative review. Postgrad Med. 2018;130:173–82. https://doi.org/10.1080/00325481.2018.1435129.
U.S. Food and Drug Administration (FDA). Questions and answers on FDA’s Adverse Event Reporting System (FAERS). U.S. Food and Drug Administration; 2018. FDA information site https://www.fda.gov/drugs/surveillance/questions-and-answers-fdas-adverse-event-reporting-system-faers. Accessed 16 Feb 2020.
Yoshimura K, Kadoyama K, Sakaeda T, Sugino Y, Ogawa O. A survey of the FAERS database concerning the adverse event profiles of α 1-adrenoreceptor blockers for lower urinary tract symptoms. 2013;10.
Sakaeda T, Tamon A, Kadoyama K, Okuno Y. Data mining of the public version of the FDA adverse event reporting system. Int J Med Sci. 2013;10:796–803.
Patel DK, Stanford FC. Safety and tolerability of new-generation anti-obesity medications: a narrative review. Postgrad Med. 2018;130:173–82.
Bate A, Evans SJW. Quantitative signal detection using spontaneous ADR reporting. Pharmacoepidemiol Drug Saf. 2009;18:427–36.
Mckinney W. Data structures for statistical computing in Python. Proceedings of the 9th Python in Science Conference. 2010;16979000(Scipy):51–6.
Seabold S, Perktold J. Statsmodels: econometric and statistical modeling with Python. Proceedings of the 9th Python in Science Conference. 2010;(Scipy):57–61.
DrugBank. Lorcaserin. 2018. https://www.drugbank.ca/drugs/DB04871. Accessed 29 May 2018.
Bohula EA, Wiviott SD, McGuire DK, Inzucchi SE, Kuder J, Im K, et al. Cardiovascular safety of lorcaserin in overweight or obese patients. N Engl J Med. 2018. https://doi.org/10.1056/NEJMoa1808721.
Pariente A, Gregoire F, Fourrier-Reglat A, Haramburu FMN. Impact of safety alerts on measures of disproportionality in spontaneous reporting databases: the notoriety bias. Drug Saf. 2007;30:891–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Gorelik, E., Gorelik, B., Masarwa, R. et al. The cardiovascular safety of antiobesity drugs—analysis of signals in the FDA Adverse Event Report System Database. Int J Obes 44, 1021–1027 (2020). https://doi.org/10.1038/s41366-020-0544-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-020-0544-4
This article is cited by
-
Phentermine in the Modern Era of Obesity Pharmacotherapy: Does It Still Have a Role in Treatment?
Current Obesity Reports (2024)
-
Unraveling the serotonin saga: from discovery to weight regulation and beyond - a comprehensive scientific review
Cell & Bioscience (2023)
-
Obesity Management in Cardiometabolic Disease: State of the Art
Current Atherosclerosis Reports (2021)