Abstract
Background/objectives
Bariatric surgery improves nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH), but the underlying mechanisms remain elusive. We evaluated the potential role of ghrelin isoforms in the amelioration of hepatic inflammation after sleeve gastrectomy and Roux-en-Y gastric bypass (RYGB).
Subjects/methods
Plasma ghrelin isoforms were measured in male Wistar rats (n = 129) subjected to surgical (sham operation, sleeve gastrectomy, or RYGB) or dietary interventions [fed ad libitum a normal (ND) or a high-fat diet (HFD) or pair-fed diet]. The effect of acylated and desacyl ghrelin on markers of inflammation, mitochondrial dysfunction, and endoplasmic reticulum (ER) stress in primary rat hepatocytes under palmitate-induced lipotoxic conditions was assessed.
Results
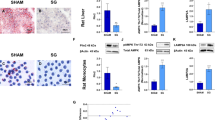
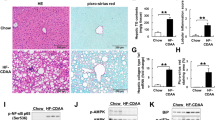
Plasma desacyl ghrelin was decreased after sleeve gastrectomy and RYGB, whereas the acylated/desacyl ghrelin ratio was augmented. Both surgeries diminished obesity-associated hepatic steatosis, CD68+- and apoptotic cells, proinflammatory JNK activation, and Crp, Tnf, and Il6 transcripts. Moreover, a postsurgical amelioration in the mitochondrial DNA content, oxidative phosphorylation (OXPHOS) complexes I and II, and ER stress markers was observed. Specifically, following bariatric surgery GRP78, spliced XBP-1, ATF4, and CHOP levels were reduced, as were phosphorylated eIF2α. Interestingly, acylated and desacyl ghrelin inhibited steatosis and inflammation of palmitate-treated hepatocytes in parallel to an upregulation of OXPHOS complexes II, III, and V, and a downregulation of ER stress transducers IRE1α, PERK, ATF6, their downstream effectors, ATF4 and CHOP, as well as chaperone GRP78.
Conclusions
Our data suggest that the increased relative acylated ghrelin levels after bariatric surgery might contribute to mitigate obesity-associated hepatic inflammation, mitochondrial dysfunction, and ER stress.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD) and European Association for the Study of Obesity (EASO). Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–402.
Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84.
Simões ICM, Fontes A, Pinton P, Zischka H, Wieckowski MR. Mitochondria in non-alcoholic fatty liver disease. Int J Biochem Cell Biol. 2018;95:93–9.
Einer C, Hohenester S, Wimmer R, Wottke L, Artmann R, Schulz S, et al. Mitochondrial adaptation in steatotic mice. Mitochondrion. 2018;40:1–12.
Koliaki C, Szendroedi J, Kaul K, Jelenik T, Nowotny P, Jankowiak F, et al. Adaptation of hepatic mitochondrial function in humans with non-alcoholic fatty liver is lost in steatohepatitis. Cell Metab. 2015;21:739–46.
Rector RS, Thyfault JP, Uptergrove GM, Morris EM, Naples SP, Borengasser SJ, et al. Mitochondrial dysfunction precedes insulin resistance and hepatic steatosis and contributes to the natural history of non-alcoholic fatty liver disease in an obese rodent model. J Hepatol. 2010;52:727–36.
Begriche K, Igoudjil A, Pessayre D, Fromenty B. Mitochondrial dysfunction in NASH: causes, consequences and possible means to prevent it. Mitochondrion. 2006;6:1–28.
Puri P, Mirshahi F, Cheung O, Natarajan R, Maher JW, Kellum JM, et al. Activation and dysregulation of the unfolded protein response in nonalcoholic fatty liver disease. Gastroenterology. 2008;134:568–76.
Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107:881–91.
Seki E, Brenner DA, Karin M. A liver full of JNK: signaling in regulation of cell function and disease pathogenesis, and clinical approaches. Gastroenterology. 2012;143:307–20.
Lassailly G, Caiazzo R, Pattou F, Mathurin P. Bariatric surgery for curing NASH in the morbidly obese? J Hepatol. 2013;58:1249–51.
Mathurin P, Hollebecque A, Arnalsteen L, Buob D, Leteurtre E, Caiazzo R, et al. Prospective study of the long-term effects of bariatric surgery on liver injury in patients without advanced disease. Gastroenterology. 2009;137:532–40.
Taitano AA, Markow M, Finan JE, Wheeler DE, Gonzalvo JP, Murr MM. Bariatric surgery improves histological features of nonalcoholic fatty liver disease and liver fibrosis. J Gastrointest Surg. 2015;19:429–36.
Ezquerro S, Méndez-Giménez L, Becerril S, Moncada R, Valentí V, Catalán V, et al. Acylated and desacyl ghrelin are associated with hepatic lipogenesis, ß-oxidation and autophagy: role in NAFLD amelioration after sleeve gastrectomy in obese rats. Sci Rep. 2016;6:39942.
Ezquerro S, Mocha F, Frühbeck G, Guzmán-Ruiz R, Valentí V, Mugueta C, et al. Ghrelin reduces TNF-α-induced human hepatocyte apoptosis, autophagy, and pyroptosis: role in obesity-associated NAFLD. J Clin Endocrinol Metab. 2019;104:21–37.
Talavera-Urquijo E, Rodríguez-Navarro S, Beisani M, Salcedo-Allende MT, Chakkur A, Arús-Avilés M, et al. Morphofunctional changes after sleeve gastrectomy and very low calorie diet in an animal model of non-alcoholic fatty liver disease. Obes Surg. 2018;28:142–51.
Tamboli RA, Antoun J, Sidani RM, Clements A, Harmata EE, Marks-Shulman P, et al. Metabolic responses to exogenous ghrelin in obesity and early after Roux-en-Y gastric bypass in humans. Diabetes Obes Metab. 2017;19:1267–75.
Frühbeck G, Díez Caballero A, Gil MJ. Fundus functionality and ghrelin concentrations after bariatric surgery. N Engl J Med. 2004;350:308–9.
Barazzoni R, Zanetti M, Nagliati C, Cattin MR, Ferreira C, Giuricin M, et al. Gastric bypass does not normalize obesity-related changes in ghrelin profile and leads to higher acylated ghrelin fraction. Obesity. 2013;21:718–22.
Mosinski JD, Pagadala MR, Mulya A, Huang H, Dan O, Shimizu H, et al. Gastric bypass surgery is protective from high-fat diet-induced non-alcoholic fatty liver disease and hepatic endoplasmic reticulum stress. Acta Physiol. 2016;217:141–51.
Sacks J, Mulya A, Fealy CE, Huang H, Mosinski JD, Pagadala MR, et al. Effect of Roux-en-Y gastric bypass on liver mitochondrial dynamics in a rat model of obesity. Physiol Rep. 2018; 6:e13600.
Porteiro B, Díaz-Ruíz A, Martínez G, Senra A, Vidal A, Serrano M, et al. Ghrelin requires p53 to stimulate lipid storage in fat and liver. Endocrinology. 2013;154:3671–9.
Sangiao-Alvarellos S, Vazquez MJ, Varela L, Nogueiras R, Saha AK, Cordido F, et al. Central ghrelin regulates peripheral lipid metabolism in a growth hormone-independent fashion. Endocrinology. 2009;150:4562–74.
Li Z, Xu G, Qin Y, Zhang C, Tang H, Yin Y, et al. Ghrelin promotes hepatic lipogenesis by activation of mTOR-PPARgamma signaling pathway. Proc Natl Acad Sci USA. 2014;111:13163–8.
Barazzoni R, Semolic A, Cattin MR, Zanetti M, Guarnieri G. Acylated ghrelin limits fat accumulation and improves redox state and inflammation markers in the liver of high-fat-fed rats. Obesity. 2014;22:170–7.
Frühbeck G, Alonso R, Marzo F, Santidrián S. A modified method for the indirect quantitative analysis of phytate in foodstuffs. Anal Biochem. 1995;225:206–12.
Bruinsma BG, Uygun K, Yarmush ML, Saeidi N. Surgical models of Roux-en-Y gastric bypass surgery and sleeve gastrectomy in rats and mice. Nat Protoc. 2015;10:495–507.
Rodríguez A, Catalán V, Becerril S, Gil MJ, Mugueta C, Gómez-Ambrosi J, et al. Impaired adiponectin-AMPK signalling in insulin-sensitive tissues of hypertensive rats. Life Sci. 2008;83:540–9.
Méndez-Giménez L, Becerril S, Moncada R, Valentí V, Ramírez B, Lancha A, et al. Sleeve gastrectomy reduces hepatic steatosis by improving the coordinated regulation of aquaglyceroporins in adipose tissue and liver in obese rats. Obes Surg. 2015;25:1723–34.
Catalán V, Gómez-Ambrosi J, Rotellar F, Silva C, Rodríguez A, Salvador J, et al. Validation of endogenous control genes in human adipose tissue: relevance to obesity and obesity-associated type 2 diabetes mellitus. Horm Metab Res. 2007;39:495–500.
Rodríguez A, Gómez-Ambrosi J, Catalán V, Gil MJ, Becerril S, Sáinz N, et al. Acylated and desacyl ghrelin stimulate lipid accumulation in human visceral adipocytes. Int J Obes. 2009;33:541–52.
Heinonen S, Buzkova J, Muniandy M, Kaksonen R, Ollikainen M, Ismail K, et al. Impaired mitochondrial biogenesis in adipose tissue in acquired obesity. Diabetes. 2015;64:3135–45.
Rodríguez A, Gómez-Ambrosi J, Catalán V, Rotellar F, Valentí V, Silva C, et al. The ghrelin O-acyltransferase-ghrelin system reduces TNF-α-induced apoptosis and autophagy in human visceral adipocytes. Diabetologia. 2012;55:3038–50.
Perks KL, Ferreira N, Richman TR, Ermer JA, Kuznetsova I, Shearwood AJ, et al. Adult-onset obesity is triggered by impaired mitochondrial gene expression. Sci Adv. 2017;3:e1700677.
Myronovych A, Kirby M, Ryan KK, Zhang W, Jha P, Setchell KD, et al. Vertical sleeve gastrectomy reduces hepatic steatosis while increasing serum bile acids in a weight-loss-independent manner. Obesity. 2014;22:390–400.
Wang Y, Liu J. Sleeve gastrectomy relieves steatohepatitis in high-fat-diet-induced obese rats. Obes Surg. 2009;19:921–5.
Yan H, Gao Y, Zhang Y. Inhibition of JNK suppresses autophagy and attenuates insulin resistance in a rat model of nonalcoholic fatty liver disease. Mol Med Rep. 2017;15:180–6.
Mao Y, Cheng J, Yu F, Li H, Guo C, Fan X. Ghrelin attenuated lipotoxicity via autophagy induction and nuclear factor-κB inhibition. Cell Physiol Biochem. 2015;37:563–76.
Mao Y, Wang J, Yu F, Li Z, Li H, Guo C, et al. Ghrelin protects against palmitic acid or lipopolysaccharide-induced hepatocyte apoptosis through inhibition of MAPKs/iNOS and restoration of Akt/eNOS pathways. Biomed Pharmacother. 2016;84:305–13.
Teodoro JS, Rolo AP, Duarte FV, Simoes AM, Palmeira CM. Differential alterations in mitochondrial function induced by a choline-deficient diet: understanding fatty liver disease progression. Mitochondrion. 2008;8:367–76.
Perfield JW 2nd, Ortinau LC, Pickering RT, Ruebel ML, Meers GM, Rector RS. Altered hepatic lipid metabolism contributes to nonalcoholic fatty liver disease in leptin-deficient ob/ob mice. J Obes. 2013;2013:296537.
Peng Y, Murr MM. Roux-en-Y gastric bypass improves hepatic mitochondrial function in obese rats. Surg Obes Relat Dis. 2013;9:429–35.
Andrews ZB, Erion D, Beiler R, Liu ZW, Abizaid A, Zigman J, et al. Ghrelin promotes and protects nigrostriatal dopamine function via a UCP2-dependent mitochondrial mechanism. J Neurosci. 2009;29:14057–65.
Barazzoni R, Gortan Cappellari G, Palus S, Vinci P, Ruozi G, Zanetti M, et al. Acylated ghrelin treatment normalizes skeletal muscle mitochondrial oxidative capacity and AKT phosphorylation in rat chronic heart failure. J Cachexia Sarcopenia Muscle. 2017;8:991–8.
Fujimura K, Wakino S, Minakuchi H, Hasegawa K, Hosoya K, Komatsu M, et al. Ghrelin protects against renal damages induced by angiotensin-II via an antioxidative stress mechanism in mice. PLoS ONE. 2014;9:e94373.
Rossetti A, Togliatto G, Rolo AP, Teodoro JS, Granata R, Ghigo E, et al. Unacylated ghrelin prevents mitochondrial dysfunction in a model of ischemia/reperfusion liver injury. Cell Death Discov. 2017;3:17077.
Barazzoni R, Bosutti A, Stebel M, Cattin MR, Roder E, Visintin L, et al. Ghrelin regulates mitochondrial-lipid metabolism gene expression and tissue fat distribution in liver and skeletal muscle. Am J Physiol Endocrinol Metab. 2005;288:E228–235.
Malhi H, Kaufman RJ. Endoplasmic reticulum stress in liver disease. J Hepatol. 2011;54:795–809.
Kammoun HL, Chabanon H, Hainault I, Luquet S, Magnan C, Koike T, et al. GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J Clin Invest. 2009;119:1201–15.
Fu S, Watkins SM, Hotamisligil GS. The role of endoplasmic reticulum in hepatic lipid homeostasis and stress signaling. Cell Metab. 2012;15:623–34.
Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–89.
Sha H, He Y, Yang L, Qi L. Stressed out about obesity: IRE1alpha-XBP1 in metabolic disorders. Trends Endocrinol Metab. 2011;22:374–81.
Jiang D, Niwa M, Koong AC. Targeting the IRE1alpha-XBP1 branch of the unfolded protein response in human diseases. Semin Cancer Biol. 2015;33:48–56.
Dara L, Ji C, Kaplowitz N. The contribution of endoplasmic reticulum stress to liver diseases. Hepatology. 2011;53:1752–63.
Jiang D, Wan F. Exendin-4 protects INS-1 cells against palmitate-induced apoptosis through the IRE1alpha-Xbp1 signaling pathway. Exp Ther Med. 2018;16:1029–35.
Lee HJ, Cui R, Choi SE, Jeon JY, Kim HJ, Kim TH, et al. Bitter melon extract ameliorates palmitate-induced apoptosis via inhibition of endoplasmic reticulum stress in HepG2 cells and high-fat/high-fructose-diet-induced fatty liver. Food Nutr Res. 2018; 62:1319.
Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–40.
Gregor MF, Yang L, Fabbrini E, Mohammed BS, Eagon JC, Hotamisligil GS, et al. Endoplasmic reticulum stress is reduced in tissues of obese subjects after weight loss. Diabetes. 2009;58:693–700.
Chung H, Chung HY, Bae CW, Kim CJ, Park S. Ghrelin suppresses tunicamycin- or thapsigargin-triggered endoplasmic reticulum stress-mediated apoptosis in primary cultured rat cortical neuronal cells. Endocr J. 2011;58:409–20.
Tian X, Liu Z, Yu T, Yang H, Feng L. Ghrelin ameliorates acute lung injury induced by oleic acid via inhibition of endoplasmic reticulum stress. Life Sci. 2018;196:1–8.
Ercan S, Kencebay C, Basaranlar G, Derin N, Aslan M. Induction of xanthine oxidase activity, endoplasmic reticulum stress and caspase activation by sodium metabisulfite in rat liver and their attenuation by Ghrelin. Food Chem Toxicol. 2015;76:27–32.
Acknowledgements
We gratefully acknowledge the valuable collaboration of Beatriz Ramírez (Metabolic Research Laboratory, Clínica Universidad de Navarra) for her technical assistance, and all the staff of the breeding house of the University of Navarra.
Funding
This work was supported by Fondo de Investigación Sanitaria-FEDER (FIS PI16/00221 and PI16/01217) from the Instituto de Salud Carlos III. SE was recipient of a predoctoral grant from the Spanish Ministerio de Educación, Cultura y Deporte (FPU15/02599). CIBEROBN is an initiative of the Instituto de Salud Carlos III, Spain.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Ezquerro, S., Becerril, S., Tuero, C. et al. Role of ghrelin isoforms in the mitigation of hepatic inflammation, mitochondrial dysfunction, and endoplasmic reticulum stress after bariatric surgery in rats. Int J Obes 44, 475–487 (2020). https://doi.org/10.1038/s41366-019-0420-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-019-0420-2
This article is cited by
-
Endoplasmic reticulum stress and therapeutic strategies in metabolic, neurodegenerative diseases and cancer
Molecular Medicine (2024)
-
Ghrelin Inhibits ACL Derived Fibroblasts Pyroptosis and Promotes Migration Through Regulating NF-κB p65/NLRP3 Signaling
International Journal of Peptide Research and Therapeutics (2023)
-
Molecular and cellular mechanisms underlying the hepatoprotective role of ghrelin against NAFLD progression
Journal of Physiology and Biochemistry (2023)
-
The outcomes of bariatric surgery on rheumatoid arthritis disease activity: a prospective cohort study
Scientific Reports (2020)