Abstract
Background:
Some of the health advantages of breast-fed as compared to formula-fed infants have been suggested to be due to metabolic programming effects resulting from early nutrition.
Methods:
In a prospective double-blinded randomized trial, 160 infants <2 mo of age were randomized to experimental formula (EF) with added milk fat globule membrane (MFGM) or standard formula (SF) until 6 mo of age. A breast-fed reference (BFR) group consisted of 80 infants. Measurements were made at inclusion and at 4, 6, and 12 mo of age.
Results:
During the intervention, the EF group had higher total serum cholesterol concentration than the SF group, reaching the level of the BFR group. The EF group had a low-density lipoprotein to high-density lipoprotein ratio not significantly different from the SF group but lower than the BFR group.
Conclusion:
Supplementation of infant formula with MFGM modified the fat composition of the formula and narrowed the gap between breast-fed and formula-fed infants with regard to serum lipid status at 12 mo.
Similar content being viewed by others
Main
The influence of early environmental factors, including nutrition, on future cardiovascular disease (CVD) risk (1) and, in a broader view, the concept of early metabolic programming and future health (2,3) have been extensively discussed during the past decades. Observational studies have suggested that formula-fed infants as compared with breast-fed infants have an elevated risk of future CVD, since they have faster early growth rate (4) and higher risk of overweight (5), type 2 diabetes (6), high blood pressure (7), and unfavorable blood lipid profile (8) in adult age. Formula-fed preterm infants have higher serum C-reactive protein (CRP) concentration, higher serum low-density lipoprotein to high-density lipoprotein (LDL:HDL) ratio, and higher serum apolipoprotein B to apolipoprotein A1 (apoB:apoA1) ratio in adolescence as compared with preterm infants receiving banked breast milk (9). Studies on cholesterol trajectories for breast-fed and formula-fed infants illustrate that breast-fed infants initially have higher total serum cholesterol concentration (s-Ch) and LDL cholesterol (LDL-Ch); in childhood the levels become equal, while in adulthood, the previously breast-fed infants have lower total s-Ch and LDL-Ch than their formula-fed counterparts (10,11). The observed average difference in total s-Ch in adulthood between previously exclusively breast-fed and non–breast-fed infants, 0.15 mmol/l, might seem trivial on an individual level. However, on a population level, it is not. Based on this difference, it has been estimated that 5% of all coronary heart disease would be avoided if all infants were exclusively breast-fed during the first 3 mo of age (8). To what degree the cholesterol content in infant formula influences s-Ch and LDL-Ch later in life is still unclear. A randomized study on infants fed a low vs. high cholesterol formula showed only minimal changes in cholesterol metabolism 6 mo after the intervention (12).
The milk fat globule membrane (MFGM) is a small but biologically active milk fraction rich in polar lipids including cholesterol and phospholipids, as well as glycoproteins (13). As bovine milk fat has not been used in infant formula, this milk fraction has historically been discarded in the manufacturing of infant formulas. Recently, commercially available MFGM-enriched bovine milk fractions have gained interest as being potentially health-promoting in several aspects (14), and studies have shown positive effects on cognitive development (15) and diarrhea (16) in infants, febrile episodes in children (17), and on blood lipids in adults (18).
This study includes secondary outcomes from a randomized controlled trial with the overall aim to evaluate health effects in infants fed an experimental formula (EF) with reduced energy and protein content and supplemented with a bovine MFGM-enriched milk fraction. Primary outcomes in the trial were weight at 6 mo and cognitive function at 12 mo and have been reported elsewhere (15). The aim of this study was to investigate the effects of the EF on serum lipids, adipokines, homocysteine, inflammatory biomarkers, and blood pressure until 12 mo of age. Our hypothesis was that infants fed EF would have outcomes more similar to breast-fed infants as compared with infants fed standard formula (SF).
Results
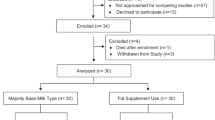
Of 240 included infants, 213 (89%) remained in the study at 12 mo ( Figure 1 ). Blood samples were available from 232 (97%), 216 (90%), 214 (89%), and 195 (81%) of the infants at baseline, 4, 6, and 12 mo of age, respectively. The fasting time (mean ± SD) before blood samples were drawn was shorter in the breast-fed reference (BFR) group as compared with the formula-fed groups at baseline (167 ± 43 vs. 183 ± 43 min; P = 0.007), 4 mo of age (160 ± 28 vs. 174 ± 38 min; P = 0.006), and 6 mo of age (159 ± 33 vs. 172 ± 39 min; P = 0.02) but not at 12 mo of age (173 ± 38 vs. 183 ± 67 min; P = 0.25). There were no significant differences in fasting time between the EF and SF groups at any age. Rates of dropout and noncompliance are shown in Figure 1 .
Infants in the study, compliance to intervention/still breast-fed (PP), and dropouts for the experimental formula (EF), standard formula (SF), and breast-fed reference (BFR) groups.
Serum Lipids
Trajectories for s-Ch, HDL-Ch, and LDL-Ch are shown in Figure 2 . In the longitudinal analysis, the EF and SF groups differed in s-Ch (P = 0.012) and HDL-Ch (P = 0.010), but not in LDL-Ch (P = 0.085). In a cross-sectional analysis, the EF group had higher s-Ch than the SF group at 4 mo (P = 0.040) and 6 mo (P = 0.027), but not at baseline (P = 0.44) or 12 mo of age (P = 0.52). The EF and SF groups did not differ significantly in cross-sectional HDL-Ch at any time point. In cross-sectional ANOVA including also the BFR group, the BFR group had higher s-Ch than the EF (P < 0.001) and SF (P < 0.001) groups at baseline (P < 0.001 and P < 0.001) and 4 mo of age (P < 0.001 and P < 0.001). At 6 mo of age, the BFR group had higher s-Ch than the SF group (P = 0.043) but not the EF group (P = 1.0), and at 12 mo of age, s-Ch did not differ among the three groups (P = 0.075). The EF and SF groups did not differ in the longitudinal analysis of LDL:HDL ratio during the intervention (P = 0.73) ( Table 1 ). The BFR group had higher LDL:HDL ratio at baseline (P < 0.001 and P < 0.001), 4 mo (P < 0.001 and P < 0.001) and 6 mo (P = 0.003 and P = 0.002), but not at 12 mo of age (P = 1.0 and P = 1.0) as compared with the EF and SF groups, respectively. There was no difference between the EF and SF groups in the longitudinal analysis of serum triglyceride concentration (s-TG) from baseline until 12 mo of age ( Table 1 ). In cross-sectional ANOVA including all three groups, the groups did not differ significantly in s-TG at baseline (P = 0.19), 6 mo (P = 0.23), or 12 mo (P = 0.087). At 4 mo of age, the BFR group had higher s-TG than the SF group (P = 0.005), but not than the EF group (P = 0.91).
Plasma cholesterol trajectories (mean and 95% confidence interval) for the experimental formula (EF, filled circles), standard formula (SF, empty circles), and breast-fed reference (BFR, filled triangles) groups. (a) Total cholesterol. The EF and SF groups differed in the longitudinal analysis from baseline to 6 mo (P = 0.012). (b) HDL cholesterol. The EF and SF groups differed in the longitudinal analysis from baseline to 6 mo (P = 0.010). (c) LDL cholesterol. The EF and SF groups did not differ in the longitudinal analysis from baseline to 6 mo (P = 0.085). HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Leptin and Adiponectin
There were no significant differences in s-leptin, s- high-molecular-weight (HMW) adiponectin, or s-leptin:fat mass ratio between the EF and SF groups at 4 mo of age ( Table 2 ). In an ANOVA including also the BFR group, there were no significant differences between the groups in s-leptin (P = 0.15), s-HMW adiponectin (P = 0.77), or s-leptin:fat mass ratio (P = 0.12).
Homocysteine, High-Sensitive CRP, and Calprotectin
During the intervention, s-homocysteine levels differed between the EF and SF groups ( Table 3 ). In a cross-sectional analysis, the EF group had lower s-homocysteine than the SF group at 4 mo (P < 0.001) and 6 mo (P < 0.001), but not at baseline (P = 0.20) or 12 mo of age (P = 0.16). In cross-sectional ANOVA including all three groups, the BFR group had higher s-homocysteine than the SF group (P = 0.002), but not than the EF group (p=0.061) at baseline. The BFR group had higher s-homocysteine than the EF group (P < 0.001 and P < 0.001), but not the SF group (P = 1.0 and P = 1.0) at both 4 and 6 mo of age. There were no significant difference among the three groups in s-homocysteine at 12 mo of age (P = 0.061). High-sensitive CRP (hsCRP) did not differ significantly between the EF and SF groups from baseline until 12 mo of age ( Table 3 ). In cross-sectional ANOVA with the EF, SF, and BFR groups, there was no difference among the three groups at baseline (P = 0.69), 4 mo (P = 0.63), 6 mo (P = 0.052), or 12 mo (P = 0.20) of age. There were no significant differences in proportion (n (%)) of infants excluded from the hsCRP analysis due to levels of ≥5 mg/l among the EF, SF, and BFR groups at baseline (0 (0), 1 (1), and 0 (0); P = 0.35), at 4 mo (5 (8), 3 (4), and 3 (5); P = 0.72), or 6 mo (7 (11), 4 (6), and 11 (19); P = 0.15). At 12 mo of age, the proportion (n (%)) of infants with hsCRP ≥ 5 was higher in the EF group (7 (12%)) compared with the SF group (0 (0%); P = 0.013) but not compared with the BFR group (1 (2%); P = 0.062). There was no difference between the EF and SF groups in fecal calprotectin from baseline until 6 mo of age ( Table 3 ). The BFR group had higher fecal calprotectin than the EF (P = 0.001) and SF (P = 0.001) groups at baseline, but there was no significant difference among the three groups at 4 mo (P = 0.085) or 6 mo (P = 0.36) of age.
Blood Pressure
Systolic and diastolic blood pressure did not differ between the EF and SF groups between baseline and 12 mo of age ( Table 4 ). In cross-sectional ANOVA, there were no significant differences among the three groups at baseline (P = 0.36 and P = 0.78), 4 mo (P = 0.90 and P = 0.67), 6 mo (P = 0.95 and P = 0.39), or 12 mo (P = 0.92 and P = 0.72) in systolic or diastolic blood pressure.
Discussion
We found that infants fed EF with higher cholesterol content than SF gradually reached higher total s-Ch. At the end of the intervention, at 6 mo of age, and 6 mo thereafter, there was no significant difference between the EF and BFR groups in total s-Ch, but the SF group had lower total s-Ch than the BFR group at all time points until 6 mo of age. It is well known from early studies that s-Ch in infancy is higher in breast-fed than that in formula-fed infants due to the higher cholesterol content in human milk (19). The difference in s-Ch disappears in childhood (11), and in adolescence and thereafter, the previously breast-fed infants have lower s-Ch than their formula-fed counterparts (10). Cholesterol metabolism seems to be affected by early nutrition in several ways. Breast feeding leads to downregulation of endogenous cholesterol synthesis via 3-hydroxy-3-methylglutaryl-coenzyme A reductase (20). In baboons, the lower cholesterol synthesis together with higher LDL receptor mRNA expression associated with breast feeding was consistent even one and a half year after weaning (21).
The LDL:HDL ratio among formula-fed infants, however, did not follow the pattern of the BFR group in this study. During the intervention, the EF group kept an LDL:HDL ratio not different from the SF group, but significantly lower than the BFR group. Breast feeding has previously been shown to be associated with high non-HDL cholesterol concentrations in infancy (22). Our findings indicate that raising the total s-Ch by increasing cholesterol intake between 2 and 6 mo in formula-fed infants does not result in a higher LDL:HDL ratio. This differs from findings in a previous study, with a smaller sample size, in which infants fed a formula with higher cholesterol showed elevated total s-Ch at 4 mo of age and a trend toward elevated LDL:HDL ratio compared with infants fed a lower cholesterol formula (12). Another study showed that infants fed a formula with higher fat energy% and a modified polyunsaturated (P) to saturated (S) fatty acid ratio with unchanged cholesterol content had higher ApoB:ApoA1 ratio, suggesting that other aspects of fat composition than cholesterol content can influence infant lipoproteins. Small differences in fat energy% (53 vs. 48%) and P:S ratio (0.44 vs. 0.46) between the EF and the SF (15) might have affected lipoprotein concentrations in this study. The impact of infant LDL:HDL ratio on later cholesterol metabolism is unclear. A previous study showed that tracking of LDL-Ch and total s-Ch was stronger than tracking of HDL-Ch from infancy to 5 y of age (23). There was no significant difference in LDL:HDL ratio at 12 mo of age among any of the groups in the present study. A longer term follow-up of total s-Ch and the LDL:HDL ratio until adolescence would be desirable since a shift from higher to lower total s-Ch and LDL-Ch from infancy to adolescence for breast-fed infants compared with formula-fed infants has been shown previously (10).
The EF and SF groups did not differ in s-TG trajectories. The BFR group had higher s-TG than the SF group at 4 mo of age, probably caused by a difference in fat intake. Previous studies have, in line with our findings, shown higher s-TG in breast-fed than that in formula-fed infants during infancy (24). There was not a significant difference between the BFR and EF groups at 4 mo. Due to the higher fat:energy ratio in the EF and the precise energy regulation of intake previously described (15), the fat intake (mean ± SD) was higher in the EF group than in the SF group during the intervention (30.2 ± 6.1 vs. 27.9 ± 5.8 g/d). Even if the difference in intake did not yield a significant difference in s-TG between the EF and SF groups, the lack of difference between the BFR and EF groups could be indicative of a narrowed gap in s-TG at 4 mo of age.
In this study, s-leptin, HMW adiponectin, and leptin relative to fat mass at 4 mo of age did not differ significantly between the EF and SF groups. The effect of feeding mode on leptin levels in infancy is contradictory, as studies have shown higher (25), equal (26), and lower (27) leptin levels in formula-fed compared to breast-fed infants during the first 4 mo of life. One possible explanation for the different findings may be different patterns of leptin concentrations by age for breast-fed and formula-fed infants during the first 6 mo (26). A role of adipokines in programming effects influencing future health has been supported by several studies. Duration of breast feeding is associated with degree of methylation of the leptin gene (28). Preterm infants fed high caloric preterm formula had higher leptin levels relative to fat mass in adolescence compared with infants fed SF or banked breast milk (29). In adolescents and adults, elevated leptin is associated with impaired arterial distensibility (30) and the leptin:adiponectin ratio to increased intima–media thickness of the common carotid artery (31). We analyzed HMW adiponectin as this has, compared with total adiponectin, been shown to better reflect obesity development in children (32). In this study, there was no effect of the intervention on adipokine concentrations at 4 mo of age.
Serum homocysteine is a pediatric risk marker for future CVD. Even moderately elevated levels indicate a higher risk (33). In this study, the EF group had lower homocysteine levels at 4 and 6 mo of age, but there was no difference at baseline or at 12 mo of age. We interpret that this difference during the intervention is attributed to the unintentional difference in folate concentration between the two formulas as the EF had higher folate content, rather than differences in metabolic regulation between infants in the EF and SF groups. The BFR group had higher homocysteine concentration than the formula-fed infants, in line with previous findings and probably caused by a lower cobalamin intake (34).
Low-grade inflammation has been shown to play a central role in the development of obesity (35), metabolic syndrome (36), and atherosclerosis (37), and an association between BMI and CRP has been shown from 3 y of age (38). Innate immune responses differ between breast-fed and formula-fed infants during the first month of life (39). Later differences in systemic inflammatory parameters have only been shown in selected groups; breast feeding was associated with higher CRP in adolescents in a follow-up study on preterm infants (9) but not in another study on term infants (40). In this study, we observed no differences in hsCRP between the EF and SF groups. The breast-fed infants had significantly higher fecal calprotectin levels at baseline compared with the formula-fed infants, in line with previous findings (41). The high concentration of fecal calprotectin in young infants has been suggested to be due to increased intestinal permeability and transepithelial migration of neutrophils at this age, possibly related to the development of the intestinal microbiota (42). The concentration of calprotectin in human milk is low (42) and whether the difference between breast-fed and formula-fed infants reflects a difference in the developing gut microbiota is still unclear.
Meta-analyses of observational studies on the association between blood pressure in adulthood and infant feeding have indicated a modest blood pressure–lowering effect of breast feeding, even if the interpretation is uncertain due to selection bias (7,43). In this study, there were no differences in blood pressure between the groups during the first 12 mo.
As reported previously, there were no differences in growth, s-insulin, and urea concentrations between the EF and SF groups. Compared with the formula-fed groups, the BFR group had slightly higher weight and length gain, and higher s-insulin and urea concentrations until 6 mo of age (15).
It should be noted that all comparisons with the BFR group are observational and unadjusted. As the main focus of the study was the comparison between the randomized formula-fed groups, we did not collect enough background data, e.g., maternal and paternal serum lipid concentrations, to make a proper adjustment for the observational part of the study. Therefore, we chose to present unadjusted values in comparisons with the BFR group.
In conclusion, this study has raised the possibility of reducing the elevated long-term risk of CVD in formula-fed infants. We have shown that increasing cholesterol intake between 2 and 6 mo of age leads to a higher total s-Ch, not different from breast-fed infants, but without shifting the LDL:HDL ratio. The difference in total s-Ch between the EF and SF groups had disappeared at 12 mo of age. In light of the previously suggested programming effect of high early s-Ch and shift in s-Ch between breast-fed and formula-fed infants during childhood (10), a longer follow-up of this study during childhood, adolescence, and adulthood might answer whether the changes in early cholesterol metabolism have yielded programming effects that influence later risk factor profile for CVD.
Methods
Subjects
As described previously (15), 160 formula-fed infants (80 girls and 80 boys) and a BFR group with 80 infants (40 girls and 40 boys), all born at Umeå University Hospital, Umeå, Sweden, were enrolled from March 2008 to February 2012. Inclusion criteria were <2 mo of age, gestational age at birth 37–42 wk, birth weight 2,500–4,500 g, and absence of chronic illness. Infants in the formula groups were exclusively formula-fed at inclusion, and infants in the BFR were exclusively breast-fed at inclusion with the intention of the mother to exclusively breast feed until 4–6 mo. All groups were recommended only small amounts (taste portions) of complementary foods between 4 and 6 mo. Formula-fed infants were stratified for sex and randomized to receive EF or SF from inclusion until 6 mo of age (15). Group allocation was blinded to parents and study staff involved in data collection until all infants had completed follow-up. Formula powder was distributed to families together with preparation instructions in identical boxes marked with code number.
Study Formula
BabySemp1 (Semper AB, Sundbyberg, Sweden) was used as SF and the EF was modified from this. The macronutrient composition of the EF and SF has been described elsewhere (15). Fat, protein, and energy contents in the EF vs. SF were 3.5 vs. 3.5 g/100 ml, 1.20 vs. 1.27 g/100 ml, and 60 vs. 66 kcal/100 ml, respectively. Cholesterol content was 8 mg/100 ml in the EF and 4 mg/100 ml in the SF. The EF was supplemented with a bovine MFGM-enriched whey protein concentrate (Lacprodan MFGM-10; Arla Foods Ingredients, Viby, Denmark). MFGM proteins from the concentrate contributed to 4% (wt: wt) of the total protein in the EF. The EF and SF differed slightly in the contents of folate (6.2 and 5.5 µg/100 ml, respectively), vitamin B12 (0.056 and 0.059 mg/100 ml), and vitamin B6 (0.22 and 0.27 µg/100 ml) concentrations. There were no differences in other vitamin or micronutrient concentrations between the EF and SF as calculated from ingredients.
Protocol and Measurements
Visits were booked at inclusion (<2 mo) and at 4, 6, and 12 mo. At each visit, anthropometric data, blood pressure, fecal samples, and blood samples were collected. At 4 mo, body fat percentage was measured using air-displacement plethysmography (PeaPod; Cosmed, Rome, Italy). Blood samples were drawn >2 h after the latest meal at each visit. HsCRP was analyzed using ELISA (R&D Systems, Minneapolis, MN). Infants with hsCRP >5 mg/l were excluded from the analysis as they were suspected to have an ongoing infection. Homocysteine, total s-Ch, HDL-Ch, and s-TG were analyzed using Vitros Chemistry HCY reagent, CHOL slides, dHDL slides, and TRIG slides on Vitros 5.1 FS (Ortho Clinical Diagnostics, Sollentuna, Sweden). LDL-Ch was calculated using Friedewald’s formula (44). Leptin in all infants, and HMW adiponectin in a randomly selected subsample of 10 boys and 10 girls from each group (EF, SF, and BFR), were analyzed using ELISA (Merck Millipore, Billerica, MA). Fecal calprotectin was analyzed using ELISA (NovaTec Immundiagnostica, Dietzenbach, Germany). Cases with fecal calprotectin over the detection limit of 2,500 mg/kg were set to 2,500 mg/kg (n = 2 BFR at baseline; n = 1 BFR at 4 m, and n = 1 SF at 6 mo of age).
Data Analysis
Statistical calculations were done using IBM SPSS Statistics Version 19 (IBM New York, NY 1989, 2010). A prestudy power analysis showed that a sample size of 63 in each group was needed to detect a difference of 0.5 SD in the main outcomes weight-for-age and cognitive score at the significance level of 0.05. For the present analysis of secondary outcomes, 0.5 SD corresponds to 0.30 mmol/l in s-Ch at 6 mo of age. Expected dropout rate was 25%, hence 80 infants were included in each group. All analyses were performed on an intention-to-treat basis. To compare outcomes of repeated measurements between the randomized groups (EF vs. SF), a linear mixed model with time and group (EF or SF) as fixed effects and time as random effect was used. For outcomes where the intervention was expected to result in a direct effect (s-Ch, HDL-Ch, LDL-Ch, HDL:LDL ratio, and s-homocysteine), the model included measurements during the intervention (baseline and 4 and 6 mo), and the data from 12 mo was compared in a cross-sectional design. For outcomes where any effect was expected to give a late effect (s-TG, hsCRP, fecal calprotectin, and blood pressure), the model included data from baseline until 12 mo of age. If the linear mixed model showed significant differences between the groups, a cross-sectional analysis at each time point was performed using independent samples t-test to further explore the difference. In outcomes where only one measurement was performed (leptin, HMW adiponectin and leptin:fat mass ratio), means were compared by independent samples t-test. Variables that were not normally distributed (s-TG, homocysteine, hsCRP, and f-calprotectin) were log transformed prior to calculations. For each outcome variable, a cross-sectional multivariate analysis including paternal and maternal BMI, chronic disease and smoking, maternal weight gain during pregnancy, and gestational diabetes (15) was performed; for s-TG, the adjusted model also included fasting time. This adjustment did not affect the results regarding differences between the EF and SF groups and is not presented. To compare the data from the randomized groups with the BFR group, an unadjusted cross-sectional ANOVA including all three groups with a Bonferroni post hoc analysis was performed.
Ethics and Study Registration
This study was approved by the Regional Ethical Review Board in Umeå. Complete oral and written information about the study was given to the parents/caregivers, and written consent was obtained from parents/caregivers of all infants before inclusion. The study was registered with number NCT00624689 at clinicaltrials.gov.
Statement of Financial Support
The study was funded by grants from Sweden’s Innovation Agency (Vinnova), the Västerbotten County Council, Sweden, and Semper AB, Sweden.
Disclosure
O.H. and B.L. are members of Hero and Semper Scientific Advisory Boards. N.T. and M.D. declare no conflict of interest.
References
Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ . Weight in infancy and death from ischaemic heart disease. Lancet 1989;2:577–80.
Hales CN, Barker DJ . Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia 1992;35:595–601.
Hattersley AT, Tooke JE . The fetal insulin hypothesis: an alternative explanation of the association of low birthweight with diabetes and vascular disease. Lancet 1999;353:1789–92.
Dewey KG . Growth characteristics of breast-fed compared to formula-fed infants. Biol Neonate 1998;74:94–105.
Harder T, Bergmann R, Kallischnigg G, Plagemann A . Duration of breastfeeding and risk of overweight: a meta-analysis. Am J Epidemiol 2005;162:397–403.
Owen CG, Martin RM, Whincup PH, Smith GD, Cook DG . Does breastfeeding influence risk of type 2 diabetes in later life? A quantitative analysis of published evidence. Am J Clin Nutr 2006;84:1043–54.
Owen CG, Whincup PH, Gilg JA, Cook DG . Effect of breast feeding in infancy on blood pressure in later life: systematic review and meta-analysis. BMJ 2003;327:1189–95.
Owen CG, Whincup PH, Kaye SJ, et al. Does initial breastfeeding lead to lower blood cholesterol in adult life? A quantitative review of the evidence. Am J Clin Nutr 2008;88:305–14.
Singhal A, Cole TJ, Fewtrell M, Lucas A . Breastmilk feeding and lipoprotein profile in adolescents born preterm: follow-up of a prospective randomised study. Lancet 2004;363:1571–8.
Owen CG, Whincup PH, Odoki K, Gilg JA, Cook DG . Infant feeding and blood cholesterol: a study in adolescents and a systematic review. Pediatrics 2002;110:597–608.
Fomon SJ, Rogers RR, Ziegler EE, Nelson SE, Thomas LN . Indices of fatness and serum cholesterol at age eight years in relation to feeding and growth during early infancy. Pediatr Res 1984;18:1233–8.
Bayley TM, Alasmi M, Thorkelson T, et al. Longer term effects of early dietary cholesterol level on synthesis and circulating cholesterol concentrations in human infants. Metabolism 2002;51:25–33.
Corredig M, Roesch RR, Dalgleish DG . Production of a novel ingredient from buttermilk. J Dairy Sci 2003;86:2744–50.
Spitsberg VL . Invited review: bovine milk fat globule membrane as a potential nutraceutical. J Dairy Sci 2005;88:2289–94.
Timby N, Domellöf E, Hernell O, Lönnerdal B, Domellöf M . Neurodevelopment, nutrition, and growth until 12 mo of age in infants fed a low-energy, low-protein formula supplemented with bovine milk fat globule membranes: a randomized controlled trial. Am J Clin Nutr 2014;99:860–8.
Zavaleta N, Kvistgaard AS, Graverholt G, et al. Efficacy of an MFGM-enriched complementary food in diarrhea, anemia, and micronutrient status in infants. J Pediatr Gastroenterol Nutr 2011;53:561–8.
Veereman-Wauters G, Staelens S, Rombaut R, et al. Milk fat globule membrane (INPULSE) enriched formula milk decreases febrile episodes and may improve behavioral regulation in young children. Nutrition 2012;28:749–52.
Ohlsson L, Burling H, Nilsson A . Long term effects on human plasma lipoproteins of a formulation enriched in butter milk polar lipid. Lipids Health Dis 2009;8:44.
Fomon SJ, Bartels DJ . Concentrations of cholesterol in serum of infants in relation to diet. AMA J Dis Child 1960;99:27–30.
Wong WW, Hachey DL, Insull W, Opekun AR, Klein PD . Effect of dietary cholesterol on cholesterol synthesis in breast-fed and formula-fed infants. J Lipid Res 1993;34:1403–11.
Mott GE, Jackson EM, DeLallo L, Lewis DS, McMahan CA . Differences in cholesterol metabolism in juvenile baboons are programmed by breast- versus formula-feeding. J Lipid Res 1995;36:299–307.
Lapinleimu H, Vükari J, Nünikoski H, et al. Impact of gender, apolipoprotein E phenotypes, and diet on serum lipids and lipoproteins in infancy. J Pediatr 1997;131:825–32.
Kallio MJ, Salmenperä L, Siimes MA, Perheentupa J, Miettinen TA . Tracking of serum cholesterol and lipoprotein levels from the first year of life. Pediatrics 1993;91:949–54.
Wu TC, Huang IF, Chen YC, Chen PH, Yang LY . Differences in serum biochemistry between breast-fed and formula-fed infants. J Chin Med Assoc 2011;74:511–5.
Petridou E, Mantzoros CS, Belechri M, et al. Neonatal leptin levels are strongly associated with female gender, birth length, IGF-I levels and formula feeding. Clin Endocrinol (Oxf) 2005;62:366–71.
Lönnerdal B, Havel PJ . Serum leptin concentrations in infants: effects of diet, sex, and adiposity. Am J Clin Nutr 2000;72:484–9.
Savino F, Nanni GE, Maccario S, Costamagna M, Oggero R, Silvestro L . Breast-fed infants have higher leptin values than formula-fed infants in the first four months of life. J Pediatr Endocrinol Metab 2004;17:1527–32.
Obermann-Borst SA, Eilers PH, Tobi EW, et al. Duration of breastfeeding and gender are associated with methylation of the LEPTIN gene in very young children. Pediatr Res 2013;74:344–9.
Singhal A, Farooqi IS, O’Rahilly S, Cole TJ, Fewtrell M, Lucas A . Early nutrition and leptin concentrations in later life. Am J Clin Nutr 2002;75:993–9.
Singhal A, Farooqi IS, Cole TJ, et al. Influence of leptin on arterial distensibility: a novel link between obesity and cardiovascular disease? Circulation 2002;106:1919–24.
Norata GD, Raselli S, Grigore L, et al. Leptin:adiponectin ratio is an independent predictor of intima media thickness of the common carotid artery. Stroke 2007;38:2844–6.
Araki S, Dobashi K, Kubo K, Asayama K, Shirahata A . High molecular weight, rather than total, adiponectin levels better reflect metabolic abnormalities associated with childhood obesity. J Clin Endocrinol Metab 2006;91:5113–6.
Groner JA, Joshi M, Bauer JA . Pediatric precursors of adult cardiovascular disease: noninvasive assessment of early vascular changes in children and adolescents. Pediatrics 2006;118:1683–91.
Hay G, Johnston C, Whitelaw A, Trygg K, Refsum H . Folate and cobalamin status in relation to breastfeeding and weaning in healthy infants. Am J Clin Nutr 2008;88:105–14.
Tam CS, Clément K, Baur LA, Tordjman J . Obesity and low-grade inflammation: a paediatric perspective. Obes Rev 2010;11:118–26.
Ford ES, Ajani UA, Mokdad AH ; National Health and Nutrition Examination. The metabolic syndrome and concentrations of C-reactive protein among U.S. youth. Diabetes Care 2005;28:878–81.
Hansson GK . Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005;352:1685–95.
Ford ES . C-reactive protein concentration and cardiovascular disease risk factors in children: findings from the National Health and Nutrition Examination Survey 1999–2000. Circulation 2003;108:1053–58.
Belderbos ME, Houben ML, van Bleek GM, et al. Breastfeeding modulates neonatal innate immune responses: a prospective birth cohort study. Pediatr Allergy Immunol 2012;23:65–74.
Vérier CM, Duhamel A, Béghin L, et al.; HELENA study group. Breastfeeding in infancy is not associated with inflammatory status in healthy adolescents. J Nutr 2011;141:411–7.
Savino F, Castagno E, Calabrese R, Viola S, Oggero R, Miniero R . High faecal calprotectin levels in healthy, exclusively breast-fed infants. Neonatology 2010;97:299–304.
Olafsdottir E, Aksnes L, Fluge G, Berstad A . Faecal calprotectin levels in infants with infantile colic, healthy infants, children with inflammatory bowel disease, children with recurrent abdominal pain and healthy children. Acta Paediatr 2002;91:45–50.
Martin RM, Gunnell D, Smith GD . Breastfeeding in infancy and blood pressure in later life: systematic review and meta-analysis. Am J Epidemiol 2005;161:15–26.
Friedewald WT, Levy RI, Fredrickson DS . Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499–502.
Acknowledgements
We are grateful to the participating infants and their parents. We thank Carina Forslund and Camilla Steinwall-Lindberg for dedicated field work, Yvonne Andersson, Carina Lagerqvist, and Catarina Lundell for excellent laboratory work and analysis. We also thank Catharina Tennefors and Lars-Börje Sjöberg (Semper AB) who contributed to the practical planning of the study and the development and manufacturing of the study products.
Author information
Authors and Affiliations
Corresponding author
PowerPoint slides
Rights and permissions
About this article
Cite this article
Timby, N., Lönnerdal, B., Hernell, O. et al. Cardiovascular risk markers until 12 mo of age in infants fed a formula supplemented with bovine milk fat globule membranes. Pediatr Res 76, 394–400 (2014). https://doi.org/10.1038/pr.2014.110
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2014.110
This article is cited by
-
Diastolic blood pressure and lipid profile in breastfed versus formula-fed infants as early indicators for CVD: a cross-sectional study
Egyptian Pediatric Association Gazette (2022)
-
Gut microbiota mediates the alleviative effect of polar lipids-enriched milk fat globule membrane on obesity-induced glucose metabolism disorders in peripheral tissues in rat dams
International Journal of Obesity (2022)
-
Effect of feeding mode on infant growth and cognitive function: study protocol of the Chilean infant Nutrition randomized controlled Trial (ChiNuT)
BMC Pediatrics (2020)
-
Metabolic phenotype of breast-fed infants, and infants fed standard formula or bovine MFGM supplemented formula: a randomized controlled trial
Scientific Reports (2019)
-
Modulation of miRNA expression in aged rat hippocampus by buttermilk and krill oil
Scientific Reports (2018)