Abstract
Introduction:
The Coulter LH780 hematology analyzer can evaluate mean neutrophil volume (MNV), conductivity (MNC), scatter (MNS), and distribution width (DW). We sought to investigate the value of volume, conductivity, and scatter (VCS) parameters in diagnosis and treatment efficacy of neonatal sepsis.
Results:
We observed significant increases in MNV, volume distribution width (VDW), conductivity distribution width (CDW), and significant decreases in MNC and MNS in septic newborns. There were significant decreases in MNV, VDW, and CDW, whereas MNC and MNS increased at the end of the treatment. Gram-negative sepsis caused higher MNV and VDW than Gram-positive sepsis.
Discussion:
This is the largest reported study seeking to determine cutoff levels of neutrophil VCS parameters in diagnosis of sepsis, and the first study in the evaluation of treatment efficacy and the effects of sepsis onset time and birth weight. We suggest that neutrophil VCS parameters and their DWs are useful both for early diagnosis and evaluation of treatment efficacy in neonatal sepsis without requirement for any extra blood collection.
Methods:
Peripheral blood samples from 304 newborns, 206 in group I (76 proven and 130 clinical sepsis) and 98 in group II (control group), were studied on diagnosis, 3rd day, and at the end of the treatment.
Similar content being viewed by others
Main
Neonatal sepsis remains an important clinical syndrome despite advances in neonatology. Early diagnosis and adequate antibiotic treatment are required because of high rates of mortality and morbidity, especially in developing countries (1). Early diagnosis of neonatal sepsis is difficult because of nonspecific signs and symptoms, and noninfectious diseases may mimic neonatal sepsis. Diagnosis is made by clinical and laboratory findings. Blood culture is the gold standard laboratory technique for the diagnosis of infection, but culture results may take 48–72 h. False-negative culture results may also occur (2). Acute-phase reactants have been used to diagnose sepsis since the 1980s. Several interleukins (ILs), tumor necrosis factor, C-reactive protein (CRP), procalcitonin, immunoglobulins, and other markers have been used in the diagnosis of sepsis (3,4). Cutoff levels of IL-6 and CRP were determined in neonatal sepsis. There is no perfect marker to diagnose sepsis and combination of these markers strengthens the diagnosis (5).
The complete blood cell count and peripheral blood smear were the most commonly ordered tests for the diagnosis of sepsis for many years. Higher white blood cell (WBC) and absolute neutrophil count and, immature/total neutrophil ratio are related to sepsis. Peripheral blood smears can give diagnostic information by identifying characteristic morphologic changes seen in reactive neutrophils and monocytes (6,7). Size of the cell; density of the nuclear chromatin; presence of the nucleolus; and the presence of toxic granulation, vacuolization, and Döhle bodies are evaluated to diagnose sepsis. Immature forms of neutrophils can be identified. However, this approach is labor-intensive and time-consuming because it requires manual examination and an experienced medical technologist. Furthermore, the results may be subjective because of human interpretation, and only a few hundred cells can be analyzed for any given sample (8).
The volume, conductivity, and scatter (VCS) technology of the Coulter LH 780 hematology analyzer (Beckman Coulter, Fullerton, CA) can obtain data from more than 8,000 WBCs using direct current impedance to measure cell volume (V) for accurate size of all cell types, radio frequency opacity to characterize conductivity (C) for internal composition of each cell, and a laser beam to measure light scatter (S) for cytoplasmic granularity and nuclear structure. It has been previously reported that VCS parameters can detect morphologic changes in immature and reactive neutrophils. This so-called VCS technology is analogous to microscopic evaluation of a peripheral blood smear but evaluates more than a microscope (9,10,11,12,13).
VCS parameters were evaluated to diagnose sepsis and mean neutrophil volume (MNV), and volume distribution width (VDW) and mean neutrophil scatter (MNS) were found useful in an adult population (11,14). There is only one study in a neonatal population and it showed that MNV and VDW were useful to diagnose neonatal sepsis (13). VCS parameters seem to be promising indicators for the diagnosis of sepsis.
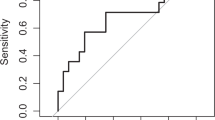
We sought to (i) investigate the value of VCS parameters in the diagnosis of neonatal sepsis, (ii) evaluate the treatment efficacy with serial measurements of VCS parameters, and (iii) determine the most appropriate cutoff value of each marker in neonatal sepsis, by using receiver operating characteristic curves and by identifying the diagnostic sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of each cutoff.
Results
We enrolled 304 patients including 76 with proven sepsis, 130 with clinical sepsis, and 98 controls in the study. The characteristics of the patients and their distribution within the groups are listed in Table 1 . Male/female ratio, gestational age, birth weight, and vaginal delivery rates were similar between groups I and II (P > 0.05). Group Ia had statistically significant lower birth weight and gestational age, and higher rate of late-onset sepsis than group Ib (P < 0.05). Twelve types of microorganism, including 43 Gram-positive and 33 Gram-negative bacteria, were isolated from blood cultures. The most frequently isolated microorganisms were Staphylococcus epidermidis (35.5%), Acinetobacter baumannii (26.3%), Klebsiella pneumoniae (17.1%), and Escherichia coli (7.9%).
Hemoglobin, WBC, and platelet counts were evaluated. WBC count was significantly higher in group I (P < 0.05) and a level above 13,150/μl was calculated as cutoff. The cutoff level for absolute neutrophil count was 7,650/μl. Platelet count was significantly lower in group I (P < 0.05) and the cutoff level for platelet count was below 286,000/μl. Mean hemoglobin levels were similar between groups (P > 0.05).
We evaluated MNV, mean neutrophil conductivity (MNC), MNS, and distribution width (DW) of these parameters. Results are listed in Table 2 . There were significant differences for MNV, VDW, MNC, conductivity distribution width (CDW), and MNS levels between the sepsis groups and the control group (P < 0.05). There was no statistical difference between sepsis subgroups for all parameters (P > 0.05).
The optimal cutoff levels for MNV, VDW, MNC, CDW, and MNS between the sepsis and control groups and also between the sepsis subgroups and control group were calculated by drawing receiver operating characteristic curves. Table 3 shows the cutoff levels. The sepsis group and sepsis subgroups had similar cutoff levels vs. the control group. The optimal cutoff levels of MNV, VDW, MNC, CDW, and MNS between groups I and II were >157.15 arbitrary units (au), >37.44 au, <159.3 au, >12.3 au, and <127.5 au, respectively. The optimal cutoff levels of IL-6 and CRP were 18.9 pg/ml and 7.6 mg/dl, respectively, in our sepsis groups. MNV, IL-6, and CRP were found to be independent risk factors of sepsis in logistic regression analysis. Sensitivity, specificity, PPV, and NPV values of MNV, VDW, MNC, CDW, MNS, IL-6, CRP, and combination of MNV, IL-6, and CRP are listed in Table 4 . Sensitivity, specificity, PPV, and NPV of MNV were 79, 82, 90, and 65%, respectively. The combination of MNV, IL-6, and CRP in the diagnosis of sepsis demonstrated sensitivity, specificity, PPV, and NPV of 94, 88, 95, and 86, respectively.
Patients with Gram-negative microorganisms had higher MNV (>163.25 au) and VDW (>41.75 au) levels than patients with Gram-positive microorganisms (P < 0.05).
We also investigated VCS parameters in the evaluation of treatment efficiency with serial measurements. Values of these parameters from sepsis work-up day to 3rd day and at the end of the treatment are listed in Table 5 . There were statistically significant decreases in MNV, VDW, and CDW levels with antibiotic treatment (P < 0.05). MNC and MNS levels increased at the end of the treatment but there was no statistical difference (P > 0.05).
Sepsis work-up day did not influence the VCS parameters. Patients with early- and late-onset sepsis had similar VCS levels (P > 0.05). Patients with birth weight below 1,500 g had higher VDW and scatter distribution width (SDW) than patients with birth weight above 1,500 g (P < 0.05).
Discussion
The automated hematology analyzers can rapidly examine very large numbers of cells to provide comprehensive hematology profiles. These parameters can be measured by the VCS technology of the Coulter LH 780 hematology analyzer (9,10,11,12,13). This so-called VCS technology is analogous to microscopic evaluation of a peripheral blood smear but evaluates more than a microscope. This technology gives the results in minutes, with no additional blood needed for the complete blood cell count.
This study, which included 76 septic neonates with positive blood cultures, 130 neonates with clinical sepsis, and 98 healthy neonates, evaluated neutrophil VCS parameters in the early diagnosis of sepsis and treatment efficacy, and sought to establish the cutoff levels, sensitivity, specificity, PPV, and NPV for each parameter. To our knowledge, this is the largest reported study seeking to determine cutoff levels of neutrophil VCS parameters in the diagnosis of sepsis and the first study investigating the neutrophil VCS parameters’ value in evaluating treatment efficacy. This study also gives additional information about effects of sepsis onset time and birth weight on VCS parameters. Previous studies mostly included adult patients and there was only one study including newborns to diagnose late-onset neonatal sepsis (11,12,13,14).
Raimondi et al. (2010) reported the cutoff levels of MNV and VDW as 148 au and 27.5 au in late-onset sepsis of very-low-birth-weight infants, including 20 with proven and 20 with clinical sepsis. The sensitivity, specificity, PPV, and NPV of MNV and VDW in this study were 95, 88, 61.3, and 98.9% and 80, 52, 25, and 92.8%, respectively (13). Combination of MNV and CRP gave sensitivity, specificity, PPV, and NPV as 95, 97, 86.4, and 99%, respectively. In addition to this small-size study, we calculated cutoff levels of MNV, VDW, MNC, CDW, and MNS. The optimal cutoff levels of MNV, VDW, MNC, CDW, and MNS between groups I and II were >157.15 au, >37.44 au, <159.3 au, >12.3 au, and <127.5 au, respectively. The optimal cutoff levels of IL-6 and CRP were 18.9 pg/ml and 7.6 mg/dl, respectively, in our study group. Sensitivity, specificity, PPV, and NPV of MNV and VDW were 79, 82, 90, and 65% and 60, 78, 85, and 48%, respectively. The combination of IL-6, CRP, and MNV in the diagnosis of sepsis gave sensitivity, specificity, PPV, and NPV of 94, 88, 95, and 86%, respectively. This combination had higher sensitivity and NPV than usage of each parameter alone. Lee et al. (2010) reported that MNC (<145.6 au) and CDW (>10.4 au) obtained from cord blood were the predictive parameters for early neonatal sepsis in newborns with chorioamnionitis (15).
Koening et al. (2010) demonstrated higher VDW in bacterial infections and higher lymphocyte VDW in viral infections in childhood (16). Chaves et al. (2005) evaluated VCS parameters in 69 adult patients with proven sepsis (11). They found higher MNV and lower MNS in the sepsis group in comparison to the control group. Cutoff levels of MNV and MNS were suggested as 150 au and 141 au, and sensitivity and specificity of MNV and MNS were 70 and 91%, and 46 and 80%, respectively. Chaves et al. (2006) evaluated VDW and they calculated its cutoff as 22 au with 79% sensitivity and 94% specificity, and as 23 au with 69% sensitivity and 100% specificity, respectively (14). Mardi et al. (2010) studied neutrophil and monocyte VCS parameters in adults including 37 sepsis and 39 nonsystemic-infection patients (12). They found higher MNV in the sepsis group than in the nonsystemic-infection and control groups, and lower MNS in the sepsis and nonsystemic-infection groups than in the control group. They designed cutoff levels of MNV as 150 au with 76% sensitivity and 63% specificity, and as 145 au with 87% sensitivity and 41% specificity, respectively. They found higher mean monocyte volume (MMV) in the sepsis group than in the nonsystemic-infection and control groups, and higher monocyte conductivity in the sepsis and nonsystemic-infection groups than in the control group. They designed cutoff levels of MMV as 170 au with 86% sensitivity and 40% specificity, and as 175 au with 60% sensitivity and 54% specificity.
Patients with Gram-negative microorganisms had higher MNV (>163.25 au) and VDW (>41.75 au) levels than patients with Gram-positive microorganisms in our study (P < 0.05). This may be associated with high cytokine production potential of Gram-negative microorganisms (17,18).
We also evaluated the value of these parameters to predict treatment efficacy. MNV, VDW, MNC, CDW, and MNS, which were useful in diagnosing neonatal sepsis, were also found to predict the treatment efficacy with serial measurements. They all tended to be within normal values during the antibiotic treatment. These parameters are evaluated without additional specimen requirements. They are quantitative, more objective, and more accurate than manual differential counts because more than 8,000 WBCs are evaluated automatically (9).
Sepsis work-up day did not influence the VCS parameters. Patients with early- and late-onset sepsis had similar VCS levels. Patients with birth weight below 1,500 g had higher VDW and SDW than patients with birth weight above 1,500 g. According to these data, neutrophil VCS parameters seem not to differ within sepsis day and birth weight and can be used to diagnose neonatal sepsis in all newborns.
Neonatal sepsis remains one of the most important clinical syndromes, especially in preterm infants, leading to high morbidity and mortality, despite advances in neonatology. Although early diagnosis and treatment is needed, because of nonspecifc signs and symptoms, difficulties and limitations of culturing procedures, and the lack of a perfect indicator of sepsis, diagnosis can be delayed. We report cut-off levels of neutrophil VCS parameters in the diagnosis of sepsis, and these parameters seem to be useful in evaluating indicators of treatment efficacy such as CRP.
In conclusion, we suggest that neutrophil VCS parameters and their DWs are useful both for early diagnosis and evaluation of treatment efficacy in neonatal sepsis without requirement for any extra blood collection.
Methods
Settings
This study was conducted at the neonatal intensive care unit of Zekai Tahir Burak Maternity Teaching Hospital, the biggest tertiary neonatology unit in Turkey, between October 2010 and April 2011. Our hospital’s birth, neonatal intensive care unit admission, and patient day numbers are approximately 20,000, 4,000, and 47,000 per year, respectively. Percentages of patients with sepsis and proven sepsis are 9.2% and 3.4%, respectively.
Participants and Definitions
Clinical findings for a diagnosis of sepsis required at least three of the following: bradycardia (<100/min), tachycardia (>200/min), hypotension, hypotonia, seizures, apnea, tachypnea, cyanosis, respiratory distress, poor skin color and perfusion, feeding difficulty, irritability, lethargy, and laboratory results showing elevated levels of IL-6 and/or CRP (19).
Patients were grouped into a sepsis group (group I), including proven and clinical sepsis, and a control group (group II): Group Ia (proven sepsis): newborns with positive blood cultures, clinical findings in agreement with the diagnosis, and elevated IL-6 and/or CRP levels during the clinical course; Group Ib (clinical sepsis): newborns with clinical findings of infection, plus a significant increase in IL-6 and/or CRP levels during the clinical course, but with negative blood cultures; Group II (control group): newborns admitted to the hospital for perinatal risk factors such as prematurity; ablatio placenta; Rh isoimmunization; conditions, such as hypoglycemia; intrauterine growth restriction; transient tachypnea; and indirect hyperbilirubinemia without clinical findings of infection were used to define control levels. Infants in the control group had normal physical examination findings and were matched as far as possible in demographic characteristics to those in the proven and clinical sepsis groups.
Techniques
Before antibiotic therapy, blood samples for VCS parameters and culture were taken from patients with a diagnosis of sepsis.
Data Collection
Data included total WBC counts, percentage of neutrophils, and the VCS parameters of neutrophils, which were generated by each individual cell passing through the aperture and were optically and electronically measured by the Coulter LH 780 (Beckman Coulter, Fullerton, CA). The reagents that are used in the LH780 to prepare the sample for the analysis allow preserving WBCs in near-native state and removing all red blood cells with the lytic solution. Volume, as measured by direct current, is used to identify the size of the cell. Conductivity, or radio frequency measurement, provides information about the internal characteristics of the cell. Light-scatter measurements, obtained as cells pass through the helium-neon laser beam, provide information about cell surface characteristics and cell granularity. The raw data events collected from volume, conductivity, and light scatter are plotted three-dimensionally on a data plot. All these measurements (mean and distribution width for volume, conductivity, and scatter) are reported as numerical values (cell population data) for each sample.
VCS parameters were evaluated on the same day of sepsis diagnosis, 3rd day, and at the end of the treatment.
Statistical Analysis
Statistical analyses were performed using the SPSS statistical package (v. 15.0). Categorical variables between groups were analyzed using the χ2 test. Comparison of means between two groups was examined by using a t test, where the data fit a normal distribution, and by Mann-Whitney U test, where the data were nonnormal distributions. For comparison of more than two groups, ANOVA was used for normal distributions and the Kruskal-Wallis test for nonnormal distributions. Receiver operating characteristic curve analysis was used to determine the power of variables to differentiate groups, and the area under the curve was calculated; significant cutoff levels were calculated using a Youden index. Logistic regression analysis was used to compare independent variables and then sensitivity and specificity were calculated for independent variables determined by logistic regression analysis. A P value of <0.05 was deemed to indicate statistical significance.
Ethics
This study was approved by the local ethics committee of Zekai Tahir Burak Maternity Teaching Hospital. An informed consent was obtained from all patients’ parents.
References
Osrin D, Vergnano S, Costello A . Serious bacterial infections in newborn infants in developing countries. Curr Opin Infect Dis 2004;17: 217–24.
Gerdes JS . Diagnosis and management of bacterial infections in the neonate. Pediatr Clin North Am 2004;51: 939–59, viii–ix.
Khassawneh M, Hayajneh WA, Kofahi H, Khader Y, Amarin Z, Daoud A . Diagnostic markers for neonatal sepsis: comparing C-reactive protein, interleukin-6 and immunoglobulin M. Scand J Immunol 2007;65: 171–5.
Dilli D, Oguz SS, Dilmen U, Köker MY, Kizilgün M . Predictive values of neutrophil CD64 expression compared with interleukin-6 and C-reactive protein in early diagnosis of neonatal sepsis. J Clin Lab Anal 2010;24: 363–70.
Celik IH, Demirel FG, Uras N, et al. What are the cut-off levels for IL-6 and CRP in neonatal sepsis? J Clin Lab Anal 2010;24: 407–12.
Mathy KA, Koepke JA . The clinical usefulness of segmented vs. stab neutrophil criteria for differential leukocyte counts. Am J Clin Pathol 1974;61: 947–58.
Wenz B, Gennis P, Canova C, Burns ER . The clinical utility of the leukocyte differential in emergency medicine. Am J Clin Pathol 1986;86: 298–303.
Pierre RV . Peripheral blood film review. The demise of the eyecount leukocyte differential. Clin Lab Med 2002;22: 279–97.
Richardson-Jones A . An automated hematology instrument for comprehensive WBC, RBC, and platelet analysis. Am Clin Lab 1990;9: 18–22.
Krause JR . Automated differentials in the hematology laboratory. Am J Clin Pathol 1990;93(4 Suppl 1): S11–6.
Chaves F, Tierno B, Xu D . Quantitative determination of neutrophil VCS parameters by the Coulter automated hematology analyzer: new and reliable indicators for acute bacterial infection. Am J Clin Pathol 2005;124: 440–4.
Mardi D, Fwity B, Lobmann R, Ambrosch A . Mean cell volume of neutrophils and monocytes compared with C-reactive protein, interleukin-6 and white blood cell count for prediction of sepsis and nonsystemic bacterial infections. Int J Lab Hematol 2010;32: 410–8.
Raimondi F, Ferrara T, Capasso L, et al. Automated determination of neutrophil volume as screening test for late-onset sepsis in very low birth infants. Pediatr Infect Dis J 2010;29: 288.
Chaves F, Tierno B, Xu D . Neutrophil volume distribution width: a new automated hematologic parameter for acute infection. Arch Pathol Lab Med 2006;130: 378–80.
Lee JC, Ahern TP, Chaves FP, Quillen K . Utility of hematologic and volume, conductivity, and scatter parameters from umbilical cord blood in predicting chorioamnionitis. Int J Lab Hematol 2010;32: 351–9.
Koenig S, Quillen K . Using neutrophil and lymphocyte VCS indices in ambulatory pediatric patients presenting with fever. Int J Lab Hematol 2010;32: 449–51.
Abe R, Oda S, Sadahiro T, et al. Gram-negative bacteremia induces greater magnitude of inflammatory response than Gram-positive bacteremia. Crit Care 2010;14: R27.
Webb SA, Kahler CM . Bench-to-bedside review: bacterial virulence and subversion of host defences. Crit Care 2008;12: 234.
Haque KN . Definitions of bloodstream infection in the newborn. Pediatr Crit Care Med 2005;6(3 Suppl): S45–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
This study was accepted and presented as a poster presentation at the 24th International Society of Laboratory Hematology (ISLH) Congress, New Orleans, LA.
Rights and permissions
About this article
Cite this article
Celik, I., Demirel, G., Aksoy, H. et al. Automated determination of neutrophil VCS parameters in diagnosis and treatment efficacy of neonatal sepsis. Pediatr Res 71, 121–125 (2012). https://doi.org/10.1038/pr.2011.16
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2011.16
This article is cited by
-
Comparison of the diagnostic accuracy of monocyte distribution width and procalcitonin in sepsis cases in the emergency department: a prospective cohort study
BMC Infectious Diseases (2022)
-
Diagnosis of neonatal sepsis: the past, present and future
Pediatric Research (2022)
-
Neutrophil Volume, conductivity and scatter (VCS) as a screening tool in neonatal sepsis
Scientific Reports (2020)
-
Utility of neutrophil volume conductivity scatter (VCS) parameter changes as sepsis screen in neonates
Journal of Perinatology (2016)
-
Elevated mean neutrophil volume represents altered neutrophil composition and reflects damage after myocardial infarction
Basic Research in Cardiology (2015)