Abstract
Knowing that IUGR is associated with altered placental transport, we aimed to characterize the placental transport of folic acid (FA), thiamine (THIAM), serotonin (5-HT), and 1-methyl-4-phenylpyridinium (MPP+) in IUGR. For this, we compared the transport characteristics of 3H-FA, 3H-THIAM, 3H-5-HT, and 3H-MPP+ in primary cultured human cytotrophoblasts isolated from IUGR and normal placentas (GRTB and NTB cells, respectively) and quantified mRNA expression of several placental transporters, by real-time RT-PCR. Our results show that GRTB cells take up 3H-FA more efficiently (higher kin and Amax values) and have higher transport capacity (higher Vmax values) for 3H-FA, 3H-5-HT, and 3H-MPP+, when compared with NTB cells. In addition, GRTB cells take up 3H-THIAM with higher affinity and 3H-MPP+ with lower affinity than NTB cells. Finally, IUGR placentas have a generalized increase in mRNA expression of FA, THIAM, 5-HT, and MPP+ transporters, when compared with normal placentas, suggesting that the increase in transport capacity may be due to increased expression of placental transporters. These results point to an effect of “compensation for the weakness” of the IUGR placenta and pose the placenta as an active mediator of the communication between maternal and fetal environments.
Similar content being viewed by others
Main
IUGR is defined as the failure of a baby to fulfill his or her growth potential in utero (1) and occurs in 4–7% of births (2). This condition is associated with an increased risk of perinatal morbidity and mortality (3) and also of developing chronic adult diseases such as cardiovascular disease and glucose intolerance (4). The notion that IUGR is due to a reduced nutritional supply from the uteroplacental unit (3) poses placental insufficiency as a primary determinant of IUGR.
The aim of this work was to compare the transport function of IUGR and normal placentas. For this, we studied the transport characteristics of several bioactive substances in primary cultured human cytotrophoblasts isolated from IUGR placentas (GRTB cells) and from normal placentas (NTB cells). The bioactive substances studied were the group B vitamins, folic acid (FA), and thiamine (THIAM), and the two organic cations, serotonin (5-HT) and 1-methyl-4-phenylpyridinium (MPP+). Furthermore, we compared the mRNA expression profile of several membrane carriers associated with the transport of these substances, in IUGR and normal placentas.
FA and THIAM are essential micronutrients for normal cellular functions, growth, and development and thus for pregnancy success. Indeed, FA deficiency during pregnancy is associated with low birth weight, increased risk of spontaneous abortion, and neural tube defects (5,6), THIAM severe deficiency during pregnancy results in reduced fetal growth, congenital malformations, and fetal death (6), and supplementation with FA during the periconceptional period reduces the incidence of low birth weight newborns and neural tube defects (5). For these reasons, the study of FA and THIAM placental transport is of major importance.
5-HT acts as a vasoconstrictor and has been implicated in the regulation of blood flow in the uteroplacental circulation (7). Finally, the low molecular weight organic cation MPP+ is considered a good model organic cation because, first, it is a very good substrate of many distinct transmembrane transporters of organic cations (see Ref. 8 for review), and second, because it is not subjected to metabolism (9). Because knowledge on placental transport of organic cations is very scarce, we decided to investigate also 5-HT and MPP+ placental transport.
Placental alkaline phosphatase (PALP, EC 3.1.3.1) is a heat-stable ecto-isoenzyme, which is expressed at high levels in placental trophoblasts (10). Its expression starts around the seventh week of pregnancy and steadily increases with duration of gestation (10), suggesting that it may be physiologically important. Alkaline phosphatase (ALP) isoenzymes have been suggested to modulate several membrane transport systems at several tissue levels (11). Indeed, protein kinases and phosphatases are involved in the reversible control of cellular phosphorylation processes, which are critical in the regulation of protein activity. Therefore, in an attempt to unravel a putative role of PALP on the modulation of placental transport, we characterized an ecto-PALP activity in NTB cells and compared it with the ecto-PALP of GRTB cells.
Our results show that GRTB cells have a higher capacity to transport 3H-FA, 3H-THIAM, 3H-5-HT, and 3H-MPP+, when compared to NTB cells, probably due to an up-regulation of transporter gene expression, suggesting that the IUGR placenta dynamically compensates for its own weakness.
MATERIALS AND METHODS
Collection of human placentas.
Collection and processing of human placentas were approved by the Ethical Committee of Hospital S. João, Porto. Human placentas were obtained at the Department of Obstetrics and Gynaecology of Hospital S. João, Porto, from uncomplicated (control, n = 11) and IUGR (n = 5) term pregnancies (37–40 wk), within half an hour after spontaneous delivery or elective caesarean section. Control placentas represented normal pregnancies with no associated maternal or fetal pathology and were collected at random. The IUGR placentas were not associated with other maternal or fetal pathology. The antepartum diagnosis of IUGR was based on ultrasonographic criteria. The diagnosis was confirmed if the birthweight was lower than the 10th percentile for gestational age, which was accurately calculated by fetal measurement on the ultrasonographic examination performed between the 11th and the 14th week of gestation. IUGR pregnancies were surveilled in our hospital and a decreased growth velocity was observed, with departure from the growth curve. All deliveries were programmed and occurred at term. Selected clinical data for normal or IUGR study groups are given in Table 1.
Primary culture of human cytotrophoblasts.
Villous cytotrophoblasts (TB cells) corresponding to control or IUGR pregnancies (NTB and GRTB cells, respectively) were isolated using a modification of the technique described by Kliman (12), as previously described by Keating et al. (13). After 72 h in culture, TB cells aggregate to form syncytial clumps corresponding to syncytiotrophoblasts and were then used for transport experiments.
To evaluate the purity of the TB cell cultures, cells in chamber slides were fixed with 4% paraformaldehyde and immunolabeled with anti-vimentin and anti-cytokeratin antibodies. Ninety-five percent of the cells were cytokeratin-positive (thus corresponding to epithelial TB cells).
In vitro experiments.
The transport experiments and the ecto-ALP assays were performed in buffer containing 125 mM NaCl, 4.8 mM KCl, 1.2 mM KH2PO4, 12.5 mM HEPES-NaOH, 12.5 mM MES, 1.2 mM MgSO4, 1.2 mM CaCl2, and 5.6 mM d(+)glucose, pH 7.5 and 7.8 (unless otherwise stated), respectively.
When tested, drugs were present during both the preincubation and incubation periods. Controls for the drugs were run in the presence of the respective solvent, which was present in a final concentration of 1% in the buffer.
Transport studies were performed as previously described (14). Incubation periods were 6 min for 3H-FA, 3H-5-HT, and 3H-MPP+, or 3 min for 3H-THIAM, unless otherwise stated. In time course experiments, 3H-FA was used at 10 nM.
Ecto-ALP assays were performed on cells grown for 96 h. The ecto-ALP reaction was carried out, after a 30-min preincubation period, with 2.5 mM pNPP, except in the kinetic experiments (as indicated) and 1 mM MgCl2. After 60 min of incubation, the reaction was stopped by adding ice-cold NaOH (0.02 M). The amount of pNP released from pNPP, reflecting ecto-ALP activity, was measured at 405 nm (adapted from Ref. 15).
RNA extraction and real-time quantitative RT-PCR.
Total RNA was extracted from NTB cells or placental villous tissue using the Tripure isolation reagent, according to manufacturer's instructions (Roche Diagnostics, Germany).
Before cDNA synthesis, total RNA was treated with DNase I (Invitrogen Corporation, CA) and 0.5 μg of resulting DNA-free RNA was reverse transcribed using Superscript Reverse Transcriptase II and random hexamer primers (Invitrogen Corporation, CA) in 20 μL of final reaction volume, according to manufacturer's instructions. Resulting cDNA was treated with RNase H (Invitrogen Corporation, CA) to degrade unreacted RNA. For the real-time quantitative PCR (qRT-PCR), 2 μL of the 20 μL reverse transcription reaction mixture was used. For the calibration curve, placental standard cDNA was diluted in five different concentrations.
Real-time PCR was carried out using a LightCycler (Roche, Nutley, NJ). Twenty microliter reactions were set up in microcapillary tubes using 0.5 μM of each primer and 4 μL of SYBR Green master mix (LightCycler FastStart DNA MasterPlus SYBR Green I, Roche). Cycling conditions were as follows: denaturation (95°C for 5 min), amplification and quantification [95°C for 10 s, annealing temperature (AT) for 10 s, and 72°C for 10 s, with a single fluorescence measurement at the end of the 72°C for 10 s segment] repeated 40 times, a melting curve program [(AT + 10)°C for 15 s and 95°C with a heating rate of 0.1°C/s and continuous fluorescence measurement], and a cooling step to 40°C. ATs, sequence of primers, primer position, and PCR product length are indicated in Table 2. Data were analyzed using LightCycler analysis software.
Immunodetection of ALP isozymes (dot-blot assay).
The detection of ALP isozyme proteins in cellular lysates of NTB cells was performed in Hybond nitrocellulose membranes using anti-TNSALP and anti-PALP antibodies. Immunodetection was accomplished with enhanced chemiluminiscence using the ECL kit.
Protein determination.
The protein content of cell monolayers was determined as described by Bradford (16), using BSA as standard.
Calculations and statistics.
For the analysis of the time course of 3H-FA transport, the parameters of eq 1 were fitted to the experimental data by a nonlinear regression analysis, using a computer-assisted method (17).

where A(t) represents the accumulation of substrate at time t, kin and kout the rate constants for inward and outward transport, respectively, and t the incubation time. Amax is defined as the accumulation at steady-state (t→∞). Kin is given in pmol · mg protein−1 · min−1 and kout in min−1. To obtain clearance values, kin was converted to μL · mg protein−1 · min−1.
For the analysis of the saturation curve of either uptake of radioactive substrates or ecto-ALP activity, the parameters of the Michaelis-Menten equation were fitted to the experimental data by a nonlinear regression analysis, using a computer-assisted method (17).
Arithmetic means are given with SEM. Statistical significance of the difference between various groups was evaluated by one-way ANOVA test followed by the Bonferroni test. For comparison between two groups, t test was used. Differences were considered to be significant when p < 0.05.
Materials.
3H-FA ([3′,5′,7,9-3H]folic acid potassium salt; specific activity 21.0 Ci · mmol−1) and 3H-5-HT (5-hydroxy[3H]tryptamine trifluoroacetate; specific activity 104 Ci · mmol−1) (Amersham Pharmacia Biotech, Buckinghamshire, UK); 3H-THIAM (thiamine HCl, [3H(G)], specific activity 10 Ci · mmol−1) and 3H-MPP+ (N-[methyl-3H]-4-phenylpyridinium acetate; specific activity 80 Ci · mmol−1) (American Radiolabeled Chemicals, Inc., St. Louis, MO); folic acid, levamisole, l-phenylalanine, MPP+ (1-methyl-4-phenylpyridinium iodide), serotonin (5-hydroxytryptamine creatinine sulfate), DIDS (4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid disodium salt), 5-MTHF (5-methyltetrahydrofolic acid disodium salt), MTX (amethopterin), pargyline hydrochloride, ascorbic acid, FBS, DMEM, HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), MES (2-[N-morpholino]ethanesulfonic acid hydrate), DNAse I, Percoll, antibiotic/antimycotic solution (100 units · mL−1 penicillin; 100 μg · mL−1 streptomycin and 0.25 μg mL−1 amphotericin B), pNPP (p-nitrophenylphosphate), pNP (p-nitrophenol), MTT (3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide) (Sigma Chemical Co., St. Louis, MO). DMSO, Triton X-100, Tris (tris-(hydroxymethyl)-aminomethane hydrochloride), trypsin (10× solution) (GIBCO, Invitrogen Corporation, Carlsbad, CA); ECL kit (Amersham Biosciences, USA); Anti-TNSALP and anti-PALP antibodies (Santacruz Biotechnology, Santacruz, CA); Hybond nitrocellulose membranes (Amersham Biosciences, USA); and Tripure isolation reagent (Roche Diagnostics, Germany).
RESULTS
Kinetics of 3H-FA, 3H-THIAM, 3H-5-HT, and 3H-MPP+ uptake by TB cells.
In this set of experiments, we determined the initial rates of 3H-FA, 3H-THIAM, 3H-5-HT, or 3H-MPP+ uptake at increasing substrate concentrations, both in NTB and GRTB cells (Fig. 1).
Kinetics of the uptake of 3H-FA (n = 8–10) (A), 3H-THIAM (n = 7–12) (B), 3H-5-HT (n = 8–9) (C), and 3H-MPP+ (n = 9–11) (D) by NTB (solid bullets) and GRTB (open bullets) cells. Cells were incubated at 37°C and pH 7.5, for 6 min, in the presence of increasing concentrations of 3H-FA (0.01–10 μM), 3H-5-HT (0.05–50 μM), 3H-MPP+ (0.2–100 μM), or for 3 min with 3H-THIAM (0.1–25 μM). Shown are arithmetic means ± SEM. *,§Significantly different from NTB cells (*p < 0.05; §p < 0.075).
Kinetic parameters of each saturation curve are shown in Table 3. Curiously, the Vmax values for all the substrates tested were 1.5–2 times higher in GRTB cells than in NTB (these differences were statistically significant for all substances except for THIAM). With respect to affinity, the Km of 3H-THIAM uptake was 2 times lower, and that of 3H-MPP+ uptake was 1.5 times higher in GRTB when compared with NTB cells. On the other hand, no differences were found between Km values for 3H-FA and 3H-5-HT uptake.
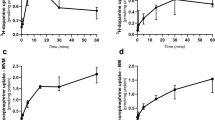
Time course of 3H-FA uptake by TB cells.
We also determined the time course of accumulation of 3H-FA in NTB and GRTB cells. For this, cells were incubated at pH 7.5 with 10 nM 3H-FA for various periods of time.
Analysis of the time course (Fig. 2) allowed the determination of values of the steady-state accumulation (Amax, 0.65 ± 0.03, n = 9, and 0.78 ± 0.03, n = 5, for NTB and GRTB cells, respectively), the rate constant of inward transport (kin, 7.16 ± 0.79, n = 9, and 22.21 ± 3.80, n = 5, for NTB and GRTB cells, respectively), and the rate constant of outward transport (kout, 0.11 ± 0.02, n = 9, and 0.28 ± 0.05, n = 5, for NTB and GRTB cells, respectively). Both the kin and the kout were significantly higher in GRTB than in NTB cells. Specifically, an amount of NTB cells corresponding to 1 mg of cell protein cleared 7.16 μL incubation medium of 3H-FA per minute, and simultaneously, 11% of intracellular 3H-FA left the cells per minute. The same amount of GRTB cells cleared 3 times more incubation medium of 3H-FA per minute and extruded as much as 28% of intracellular 3H-FA from the cells per min. As a result, the Amax for 3H-FA was significantly higher in GRTB than in NTB cells.
Ecto-ALP activity in NTB cells: dependence on culture time and on extracellular pH.
Ecto-ALP activity was quantified in NTB cells cultivated for various periods of time. Ecto-ALP activity increased for up to 2 d of culture and thereafter remained unchanged for up to 4 d of culture (Fig. 3A). l-phenylalanine (PHE; 2 mM), an inhibitor of the placental isoenzyme of ALP (PALP), inhibited ecto-ALP activity from culture d 2–4. Unexpectedly, levamisole (LEV; 1 mM), an inhibitor of the tissue nonspecific isoenzyme of ALP (TNSALP), also reduced ecto-ALP activity from culture day 1–4. Nevertheless, the ratio of inhibition in the presence of PHE or LEV (PHE/LEV) remained unchanged with culture time (Fig. 3A), indicating that the type of ecto-ALP present does not seem to change. On the basis of this information, for practical reasons, ecto-ALP assays were performed in cells cultivated for 4 d.
Dependence of ecto-ALP activity in NTB cells in relation to: culture time (A) and pH (B), and effect of ecto-ALP inhibitors. (A) Cells were cultivated for various periods of time and then incubated for 60 min with 2.5 mM pNPP in the presence of l-phenylalanine (PHE 2 mM; n = 4; ▪), levamisole (LEV 1 mM; n = 5; □), or the respective solvent (C; n = 7; ○), at pH 7.8; ×: relative contribution of ecto-ALP activities under the influence of PHE or LEV (PHE/LEV); (B) cells were incubated for 60 min with 2.5 mM pNPP at increasing pH values (7.5–9.0) in the presence of l-phenylalanine (PHE 2 mM; n = 6; ▪) or the respective solvent (C; n = 6; □). Shown are arithmetic means ± SEM. *,§Significantly different from control or day 1, respectively, (p < 0.05).
Not surprisingly, ecto-ALP activity was found to be pH-dependent (Fig. 3B), reaching its maximum at pH 9.0. In addition, PHE 2 mM inhibited ALP activity from pH 7.8 to 9.0. Cell viability was not compromised when cells were exposed to increasing pH values (up to 9.0; data not shown). Therefore, in subsequent experiments, ecto-ALP activity was determined at pH 7.8, which is a pH not very different from the physiologic pH and at which the enzyme activity can be easily measured.
In these series of experiments, we studied the variation of ecto-ALP activity with increasing substrate concentrations (pNPP, 0.1–10 mM), both in NTB and GRTB cells (Fig. 4). Ecto-ALP activity was found to be saturable in both cell types. Interestingly, Vmax value of ecto-ALP activity in NTB cells was higher, and Km value lower, than in GRTB cells.
ALP isoenzyme detection.
The finding that ecto-ALP activity was inhibited by LEV led us to investigate the presence of TNSALP in NTB cells. Therefore, we performed RT-PCR with total RNA from NTB cells and dot-blot assay with NTB cell lysates.
NTB cells expressed PALP, but not TNSALP mRNA (data not shown). This result was confirmed by immunodetection of both isoenzymes: NTB cells were positive for PALP but negative for TNSALP (Fig. 5).
Quantification of mRNA expression in normal and growth-restricted placentas.
To understand whether the observed increase in transport activity in GRTB cells was due to an increase in the transcription of membrane transporters in IUGR placentas, we quantified the mRNA levels of several transporters in normal (control, NP) and growth-restricted (GRP) placental villous tissue, by qRT-PCR. The genes encoding the following transporters were quantified: the reduced folate carrier (RFC1), the folate receptor alpha (FRα), and the proton-coupled folate transporter (PCFT), which are known to be involved in FA placental transport (14,18,19); the THIAM transporter type 1 (ThTr1) (20), which is thought to be involved in THIAM placental transport; the serotonin transporter (SERT), which has been suggested to participate in THIAM (21) and MPP+ (8) transport in BeWo and JAR cells, respectively, and is known to be involved in the placental uptake of 5-HT (22); and the organic cation transporter type 3 (OCT3), which is known to be involved in MPP+ transport in human placental basal membrane vesicles (23). Because we also investigated a putative involvement of PALP in placental transport, we also quantified the expression levels of PALP in both NP and GRP.
As shown in Fig. 6, a generalized (although not statistically significant) increase in the mRNA levels of all the studied genes in GR placentas relative to normal controls was observed.
mRNA levels of reduced folate carrier (RFC1), folate receptor alpha (FRα), proton-coupled folate transporter (PCFT), thiamine transporter type 1 (ThTr1), serotonin transporter (SERT), organic cation transporter type 3 (OCT3), and placental alkaline phosphatase (PALP) in normal (NP, control taken as 100%, n = 4; ▪) and growth-restricted (GRP, n = 5; □) placental villous tissue determined by qRT-PCR. Shown are arithmetic means ± SEM corresponding to the expression of each test gene relative to β-actin.
DISCUSSION
The aim of this work was to compare the transport characteristics of several bioactive substances (the vitamins FA and THIAM, and the organic cations 5-HT and MPP+) in primary cultured human cytotrophoblasts isolated from IUGR (GRTB cells) and control placentas (NTB cells).
Human primary cultured cytotrophoblasts (TB cells) are recognized as a suitable cell model to study human placental transport and function (24) because villous cytotrophoblast cells in culture spontaneously differentiate into a functional and polarized syncytiotrophoblast-like structure that resembles the in vivo syncytiotrophoblast (25,26).
From the kinetic analysis, we conclude that GRTB cells have a higher transport capacity (higher Vmax) both for the vitamins and for the organic cations tested, when compared with NTB cells. Moreover, we observed that GRTB cells take up 3H-THIAM with greater affinity (lower Km) than NTB cells. The time course analysis of 3H-FA uptake reinforces the kinetic results, demonstrating that GRTB cells are indeed more efficient in taking up and accumulating this vitamin, when compared with their normal counterparts.
To our knowledge, this is the first report showing changes in transport of FA, THIAM, 5-HT, and MPP+ in IUGR. Given the physiologic importance of these particular substances in pregnancy (FA and THIAM as obligatory vitamins for fetal development, 5-HT as a placental vasoconstrictor, and MPP+ as a model for organic cation), the results of this study are with no doubt important.
IUGR has already been shown to be associated with altered placental transport of some substances (3). In that context, it has been hypothesized that down-regulation of placental transporters in IUGR may correspond to a primary event or cause in the onset of this pathology and that the up-regulation may be secondary or compensatory for growth restriction (3). Our results are in perfect agreement with this hypothesis. Indeed, we here detect a higher transport activity in GRTB cells for all the bioactive substances tested, when compared with NTB cells (for 3H-THIAM, this increase was evident although statistically not significant), and we suggest that this compensation in activity may result from an up-regulation in transporter gene expression.
We also characterized an ecto-PALP activity in NTB cells and compared it with the ecto-PALP assayed in GRTB cells. We demonstrated for the first time an ecto-ALP activity in this cell model. This activity was shown to be inhibited by PHE, but unexpectedly, it was more potently inhibited by LEV, an inhibitor of TNS-ALP. However, NTB cells do not express TNS-ALP. Therefore, it seems that LEV inhibits PALP. Moreover, we verified that the enzyme activity is saturable and has a Km value identical to that found for the purified form of PALP (27). This observation, together with the results from the characterization described above, reinforces the idea that the ecto-ALP activity detected in this study is indeed due to PALP.
Quantification of ecto-ALP activity in GRTB cells showed that it is reduced and has a lower affinity for the substrate when compared with NTB cells. This finding is in strong agreement with the observation that serum levels of PALP are lowered in pregnancies complicated by IUGR (28) and may result from a reduced villous surface area seen in growth-restricted placentas (29). Reinforcing this association is the demonstration by Calhau et al. (11) that there is a positive correlation between ALP activity and the extent of exchange surfaces.
We observed, by qRT-PCR analysis, that PALP mRNA levels are increased in GRP when compared with NP. Although this finding may seem unexpected, it is in agreement with the increased differentiation observed by Newhouse et al. (30) in IUGR trophoblast cultures. The disparity between ecto-PALP activity and mRNA levels suggests that there may be an increased transcription of PALP in IUGR, but a defective translation or inefficient recycling of the enzyme to the cell surface. Indeed, PALP is an ecto-enzyme, which is subjected to recycling between intracellular compartments and the cell surface (15).
In conclusion, our results show that GRTB cells have higher capacity to transport 3H-FA, 3H-THIAM, 3H-5-HT, and 3H-MPP+, probably due to an up-regulation of transporter gene expression. These observations point to an effect of “compensation for the weakness” of the growth-restricted placenta and pose the placenta as an active mediator of the communication between maternal and fetal environments.
Our results also show that GRTB cells have lower ecto-PALP activity when compared with NTB cells; further work aimed at studying a putative involvement of ecto-PALP on the modulation of placental transport, as demonstrated for other ALP isoenzymes, is therefore warranted (11).
Abbreviations
- 5-HT:
-
serotonin
- FA:
-
folic acid
- GRTB:
-
primary cultured growth-restricted human cytotrophoblasts
- MPP+:
-
1-methyl-4-phenylpyridinium
- NTB:
-
primary cultured normal human cytotrophoblasts
- PALP:
-
placental alkaline phosphatase
- THIAM:
-
thiamine
References
Monk D, Moore GE 2004 Intrauterine growth restriction—genetic causes and consequences. Semin Fetal Neonatal Med 9: 371–378
McCarthy C, Cotter FE, McElwaine S, Twomey A, Mooney EE, Ryan F, Vaughan J 2007 Altered gene expression patterns in intrauterine growth restriction: potential role of hypoxia. Am J Obstet Gynecol 196: 70.e1–70.e6
Cetin I, Foidart JM, Miozzo M, Raun T, Jansson T, Tsatsaris V, Reik W, Cross J, Hauguel-de-Mouzon S, Illsley N, Kingdom J, Huppertz B 2004 Fetal growth restriction: a workshop report. Placenta 25: 753–757
Barker DJ 1998 In utero programming of chronic disease. Clin Sci (Lond) 95: 115–128
Picciano MF 2003 Pregnancy and lactation: physiological adjustments, nutritional requirements and the role of dietary supplements. J Nutr 133: 1997S–2002S
Worthington-Roberts B 1999 Nutrition. In: Cohen W, Cherry S, Merkatz I (eds) Cherry and Merkatz's Complications of Pregnancy. Lippincott Williams & Wilkins, London; pp 17–49
Bolte AC, van Geijn HP, Dekker GA 2001 Pathophysiology of preeclampsia and the role of serotonin. Eur J Obstet Gynecol Reprod Biol 95: 12–21
Martel F, Keating E 2003 Uptake of 1-methyl-4-phenylpyridinium (MPP+) by the JAR human placental choriocarcinoma cell line: comparison with 5-hydroxytryptamine. Placenta 24: 361–369
Sayre LM 1989 Biochemical mechanism of action of the dopaminergic neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Toxicol Lett 48: 121–149
Leitner K, Szlauer R, Ellinger I, Ellinger A, Zimmer KP, Fuchs R 2001 Placental alkaline phosphatase expression at the apical and basal plasma membrane in term villous trophoblasts. J Histochem Cytochem 49: 1155–1164
Calhau C, Hipolito-Reis C, Azevedo I 1999 Alkaline phosphatase and exchange surfaces. Clin Biochem 32: 153–154
Kliman HJ, Nestler JE, Sermasi E, Sanger JM, Strauss JF 3rd 1986 Purification, characterization, and in vitro differentiation of cytotrophoblasts from human term placentae. Endocrinology 118: 1567–1582
Keating E, Goncalves P, Lemos C, Costa F, Campos I, Smith SB, Bridges CC, Martel F 2007 Progesterone inhibits folic acid transport in human trophoblasts. J Membr Biol 216: 143–152
Keating E, Lemos C, Goncalves P, Martel F 2008 Acute and chronic effects of some dietary bioactive compounds on folic acid uptake and on the expression of folic acid transporters by the human trophoblast cell line BeWo. J Nutr Biochem 19: 91–100
Anagnostou F, Plas C, Forest N 1996 Ecto-alkaline phosphatase considered as levamisole-sensitive phosphohydrolase at physiological pH range during mineralization in cultured fetal calvaria cells. J Cell Biochem 60: 484–494
Bradford MM 1976 A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254
Muzyka A, Tarkany O, Yelizanof V, Sergienko U, Boichuk A 2005 Non-linear regression (curve fit), in GraphPad Prism (version 4.03) for Windows. GraphPad Software, San Diego, CA
Ganapathy V, Smith SB, Prasad PD 2004 SLC19: the folate/thiamine transporter family. Pflugers Arch 447: 641–646
Qiu A, Jansen M, Sakaris A, Min SH, Chattopadhyay S, Tsai E, Sandoval C, Zhao R, Akabas MH, Goldman ID 2006 Identification of an intestinal folate transporter and the molecular basis for hereditary folate malabsorption. Cell 127: 917–928
Dutta B, Huang W, Molero M, Kekuda R, Leibach FH, Devoe LD, Ganapathy V, Prasad PD 1999 Cloning of the human thiamine transporter, a member of the folate transporter family. J Biol Chem 274: 31925–31929
Keating E, Lemos C, Azevedo I, Martel F 2006 Characteristics of thiamine uptake by the BeWo human trophoblast cell line. J Biochem Mol Biol 39: 383–393
Nguyen TT, Tseng YT, McGonnigal B, Stabila JP, Worrell LA, Saha S, Padbury JF 1999 Placental biogenic amine transporters: in vivo function, regulation and pathobiological significance. Placenta 20: 3–11
Sata R, Ohtani H, Tsujimoto M, Murakami H, Koyabu N, Nakamura T, Uchiumi T, Kuwano M, Nagata H, Tsukimori K, Nakano H, Sawada Y 2005 Functional analysis of organic cation transporter 3 expressed in human placenta. J Pharmacol Exp Ther 315: 888–895
Sastry BV 1999 Techniques to study human placental transport. Adv Drug Deliv Rev 38: 17–39
Bloxam DL, Bax CM, Bax BE 1997 Culture of syncytiotrophoblast for the study of human placental transfer. I. Isolation and purification of cytotrophoblast. Placenta 18: 93–98
Moreau R, Simoneau L, Lafond J 2001 Characteristics of calcium uptake by BeWo cells, a human trophoblast cell line. Placenta 22: 768–775
Komoda T, Hokari S, Sonoda M, Sakagishi Y, Tamura T 1982 l-Phenylalanine inhibition of human alkaline phosphatases with p-nitrophenyl phosphate as substrate. Clin Chem 28: 2426–2428
Holmgren PA, Stigbrand T, Damber MG, von Schoultz B 1979 Serum levels of placental alkaline phosphatase in high-risk pregnancies. Obstet Gynecol 54: 631–634
Mayhew TM, Manwani R, Ohadike C, Wijesekara J, Baker PN 2007 The placenta in pre-eclampsia and intrauterine growth restriction: studies on exchange surface areas, diffusion distances and villous membrane diffusive conductances. Placenta 28: 233–238
Newhouse SM, Davidge ST, Winkler-Lowen B, Demianczuk N, Guilbert LJ 2007 In vitro differentiation of villous trophoblasts from pregnancies complicated by intrauterine growth restriction with and without pre-eclampsia. Placenta 28: 999–1003
Acknowledgements
We thank Professor Seth Guller (Yale University, New Haven, CT, USA) for technical support in establishing primary cultures; Dr. Joana Marques (University of Porto, Portugal) for β-actin primers donation; Dr. Otília Brandão (University of Porto, Portugal) for technical help with GR placentas; and Mrs. Luisa Vasques (University of Porto, Portugal) for laboratory support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the FCT and Programa Ciência, Tecnologia e Inovação do Quadro Comunitário de Apoio (POCTI/SAU-FCF/59382/2004).
Rights and permissions
About this article
Cite this article
Keating, E., Gonçalves, P., Costa, F. et al. Comparison of the Transport Characteristics of Bioactive Substances in IUGR and Normal Placentas. Pediatr Res 66, 495–500 (2009). https://doi.org/10.1203/PDR.0b013e3181b9b4a3
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/PDR.0b013e3181b9b4a3