Abstract
Cardiac output can be measured using a modified carbon dioxide Fick (mCO2F) method. A validation study was performed comparing mCO2F method–derived cardiac output (QmCO2F) with invasively measured pulmonary blood flow. In seven randomly bred ventilated newborn lambs, cardiac output was manipulated by creating hemorrhagic hypotension. When steady state was reached, QmCO2F was measured. Gas analysis was performed in simultaneously obtained arterial and venous blood samples (right atrium [RA], superior vena cava [SVC], and inferior vena cava [IVC]). Carbon dioxide exchange and pulmonary blood flow was measured continuously using a CO2SMO Plus monitor and a pulmonary ultrasonic flow probe (Qufp), respectively. Mean bias, defined as QmCO2F − Qufp, was small (respectively, −0.082 L·min−1, −0.085 L·min−1 and −0.183 L·min−1 for venous sampling from RA, SVC, and IVC). The limits of agreement were −0.328 to 0.164 L·min−1 (RA), −0.335 to 0.165 L·min−1 (SVC), and 0.415 to 0.049 L·min−1 (IVC). In conclusion, measurement of cardiac output with the mCO2F method is reliable and easily applicable in ventilated newborn lambs. For clinical use, the site of venous blood sampling is of minor importance.
Similar content being viewed by others
Main
Monitoring cardiac output is essential in the treatment of critically ill patients. It may improve the indication and choice of treatment in a hemodynamically unstable patient. Furthermore, the response to the intervention can be monitored and evaluated.
Bedside cardiac output monitoring is feasible in adult and pediatric patients, but remains complicated in the newborn. Several techniques for cardiac output monitoring in children are available (1–3). Most techniques for cardiac output measurement are not feasible in newborns. In critically ill neonates, cardiac output is usually estimated from the interpretation of several clinical variables, such as blood pressure, urine output, blood gas analysis, and capillary refill. Tibby and colleagues (4) showed that clinicians using these indirect parameters of cardiac performance were unable to predict the actual cardiac output in ventilated children and infants. The same was shown in ventilated adults (5). Nonetheless, this clinical estimation of cardiac output is nowadays still the most frequently used method. This emphasizes the need for a reliable, (semi)continuous, preferably noninvasive, accurate method of cardiac output monitoring in critically ill newborns. Only in this way can objective hemodynamic parameters be obtained on which a rational therapeutic regimen can be based.
Theoretically, cardiac output can be measured in ventilated patients using a mCO2F method. We performed a validation study of this technique in a lamb model.
METHODS
We compared the QmCO2F with measured pulmonary blood flow using a perivascular ultrasonic flow probe (ufp) positioned around the common pulmonary artery (Qufp).
mCO2F method.
The mCO2F method is based on the principle that in steady state, carbon dioxide production in tissue (CO2P) equals pulmonary carbon dioxide exchange (V˙CO2.V˙CO2 can be measured in a ventilated patient using a computer-aided analysis of expiratory airflow (Qexp) and carbon dioxide fraction in expiratory air (FeCO2).

where V˙CO2 = carbon dioxide exchange (L/min); Qexp = expiratory airflow (L/min); FeCO2 = carbon dioxide fraction in expiratory air (gradient); T = time (min).
CO2P is the product of cardiac output (Q) and the venoarterial difference in carbon dioxide concentration (C(v-a)CO2).

where CO2P = carbon dioxide production (mL/min); Q = cardiac output (L/min); CvCO2 = venous carbon dioxide concentration (mL/L); CaCO2 = arterial carbon dioxide concentration (mL/L).
Carbon dioxide concentration in blood can be measured using different methods, depending on the calculation method of carbon dioxide concentration in the erythrocyte (6–10). In this study, we used the Douglas equation (10):

where cpCO2 = total carbon dioxide concentration in plasma (mL/100 mL); cbCO2 = total carbon dioxide concentration in blood (mL/100 mL); Hb = hemoglobin concentration (g/dL); sO2 = oxygen saturation (gradient).
Total carbon dioxide concentration in plasma is calculated using the Henderson-Hasselbalch equation:

where cpCO2 = total carbon dioxide concentration in plasma (mL/100 mL); 2.226 = conversion factor mEq to mL/100 mL; s = solubility coefficient of carbon dioxide in plasma (mEq/mm Hg); pK′ = apparent pK; pCO2 = partial carbon dioxide pressure (mm Hg).

where s = solubility coefficient of carbon dioxide in plasma (mEq/mm Hg); T = temperature (°C)

For use in calculations, CO2P and V˙CO2 need to be converted to standard temperature pressure, dry (STPD) conditions.

where CO2 P STPD = carbon dioxide production under STPD conditions; CO2 P BTPS = carbon dioxide production under BTPS conditions; BTPS = body temperature pressure, saturated; T0 = standard temperature (273 K); T BTPS = temperature under BTPS (K); P BTPS = pressure under BTPS conditions (kPa); pH2O = partial pressure of water vapor at T BTPS (kPa); P0 = standard pressure (101.4 kPa).

where V˙CO2 STPD = pulmonary carbon dioxide exchange under STPD conditions; V˙CO2ATPS = pulmonary carbon dioxide exchange under ATPS conditions; ATPS = ambient temperature pressure, saturated; T0 = standard temperature (273 K); T ATPS = ambient temperature (K); P ATPS = pressure under ATPS conditions (kPa); pH2O = partial pressure of water vapor at T ATPS (kPa); P0 = standard pressure (101.4 kPa).
Then, cardiac output can be calculated using the following equation.

Q = cardiac output (L/min); (V˙CO2) STPD = pulmonary carbon dioxide exchange under STPD conditions (mL/min);
(C(v-a)CO2)STPD = venoarterial difference in carbon dioxide concentration under STPD conditions (mL/L).
Animal preparation.
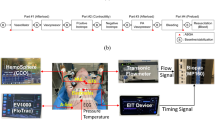
The study was approved by the Ethical Committee on Animal Research of the Radboud University Nijmegen and performed in seven randomly bred newborn lambs (2.9–6.4 kg). After anesthesia induction using midazolam (2.0 mg·kg−1) and pentobarbital (15–20 mg·kg−1), the lambs were orotracheally intubated with a cuffed endotracheal tube. The cuff was inflated to a standard pressure. It was verified by auscultation that there was no air leak around the cuffed tube. Animals were artificially ventilated with a Babylog 8000 Plus ventilator (Dräger Medizintechnik, Lübeck, Germany) using pressure control. Anesthesia was maintained with fentanyl (loading dose, 0.2 mg·kg−1; maintenance dose, 0.2 mg·kg−1·h−1) and midazolam (0.3 mg·kg−1·h−1), and muscle paralysis was performed using pancuronium (loading dose, 0.1 mg·kg−1; maintenance dose, 0.02 mg·kg−1·h−1). Ventilator settings were adjusted to maintain normoxaemia (Pao2 12–15 kPa; Sao2 90%–95%) and normocapnia (Paco2 4.0–5.5 kPa). A servocontrolled heating mattress was used to maintain the rectal temperature between 38° and 40°C. In clinical practice, it is difficult to position the tip of a central venous catheter exactly in the RA. To be able to evaluate the effect of different venous sample sites on the accuracy of the mCO2F method, we surgically inserted intravascular lines with the tip positioned in the RA, the IVC, and SVC, respectively. The position of the tip of the catheters was estimated using the registered pressure waves during insertion. The final position of the catheters was verified by postmortem evaluation. An intra-arterial catheter was inserted in the abdominal aorta through the femoral artery. A left-sided thoracotomy was performed, and after cautious preparation and identification of major structures, the ductus arteriosus was ligated. This was done to exclude any possible influence of a ductal shunt (left to right and/or right to left) on the measurement. An adequately sized ultrasonic flow probe (Transonic Systems Inc., Ithaca, NY) was placed around the pulmonary trunk.
Experimental protocol.
Measurement of the pulmonary blood flow (Qufp) was considered the gold standard because it is equal to systemic flow, when any shunts are excluded. Measurement of aortic flow will underestimate the systemic flow because the coronary flow will be missed.
After a 30-min period of stabilization, cardiac output was reduced by creating hemorrhagic hypotension. Blood was withdrawn in a stepwise manner to obtain a decrease of mean arterial blood pressure (MABP) of 10 mm Hg. After each reduction in blood pressure, a 15-min stabilization period ensured steady state. Steady state was assumed when carbon dioxide exchange was stable for at least 6 min. The mCO2F method–derived cardiac output was measured before manipulating cardiac output and after each stabilization period. Blood samples were taken from the different sampling sites for blood gas analysis, i.e. arterial blood from the abdominal aorta and venous blood from the RA, SVC, and IVC. Gas analysis of the blood samples was done immediately after sampling using a Synthesis 25 analyzer (Instrumentation Laboratory, Barcelona, Spain). The stated 90% confidence interval is ±2 mm Hg for Pco2, ±10 mpH for pH, ±0.25 mmol/L for Hb concentration and ±1.5% for oxygen saturation. Carbon dioxide exchange was measured using the CO2SMO Plus Respiratory Profile Monitor (Model 8100, Novametrix Medical Systems Inc., Wallingford, CT). According to the manufacturer, the bias of carbon dioxide measurement is −0.8% with a precision of ±3.6% (11). At the time of measurement, the following data were recorded: atmospheric pressure (Patm), positive end-expiratory pressure level, systolic arterial blood pressure (SABP), MABP, diastolic arterial blood pressure (DABP), rectal temperature (Trectal), and pulmonary blood flow via the Transonic flow probe (Qufp).
At the end of the experiment, the lamb was killed and the positions of the intravascular catheters were verified by autopsy.
Statistical analysis.
The QmCO2F was calculated using the results of blood gas analysis from the three different venous sample sites, respectively, RA, SVC, and IVC.
To evaluate the applicability of the mCO2F method in monitoring the trend of cardiac output, correlation coefficients were assessed between QmCO2F and Qufp. Bias, defined as QmCO2F − Qufp, was calculated for each measurement. For every venous sampling site, the mean bias with standard deviation (SD) was calculated. A t test was used to determine whether the bias was significantly different from zero. Bland-Altman plots were used to assess the agreement between cardiac output measurement using the mCO2F method versus the Qufp (12).
RESULTS
Table 1 shows the major hemodynamic data for each lamb at the start and the end of the experiment. Also the number of measurements per lamb and the total amount of blood withdrawn during the experiment are shown.
In all the lambs, a correct position of the intravascular catheters was observed during autopsy at the end of the experiment.
Agreement and correlation of cardiac output measurement obtained by the mCO2F method and Qufp for the three different venous sample sites are listed in Table 2. The mean bias was lowest with venous blood from the RA or SVC. The biases were statistically significant different from zero (p < 0.01). Bland-Altman plots for the three different venous sample sites are shown in Figures 1 to 3. There was no significant relationship between the bias and cardiac output.
DISCUSSION
This study shows that cardiac output can be measured reliably in ventilated newborn lambs using the mCO2F method.
Monitoring cardiac function in neonates is difficult as there are no clinical parameters (solitary or in combination) that reliably predict cardiac output (4). This illustrates the need for an accurate method of cardiac output monitoring. Objective data reflecting the hemodynamic status of a critically ill newborn may lead to a more rational treatment.
In 1870, Adolph Fick (13) described a method for cardiac output measurement using the principle of preservation of oxygen. Cardiac output can be calculated using oxygen consumption and arteriovenous oxygen concentration difference. This principle can be modified by using carbon dioxide exchange and venoarterial carbon dioxide concentration difference. In several methods of noninvasive cardiac output monitoring, the Fick principle is used. In the majority of these Fick-derived noninvasive methods, oxygen consumption is estimated by measuring carbon dioxide exchange and calculating oxygen consumption from an assumed respiratory quotient (RQ). In general the direct oxygen Fick method is regarded as the gold standard in cardiac output monitoring. We preferred a modified carbon dioxide Fick method, because this does not require any specific additional equipment, like a metabolic monitor. Rebreathing techniques are not safely applicable in newborns, because of its potential effect on cerebral blood flow by a fluctuating partial carbon dioxide pressure in blood (14). Several studies show that modified carbon dioxide methods are at least as accurate as the oxygen Fick method (15–17).
From all published methods for calculating total carbon dioxide concentration in blood, we selected the Douglas equation (10). Unfortunately, it is not known whether this algorithm is also applicable to fetal and/or neonatal blood.
In this study, the ductus arteriosus was ligated to exclude any influence of a ductal shunt on the measurement of pulmonary blood flow. Theoretically, the carbon dioxide Fick equation for calculating systemic blood flow should not be influenced by any intracardiac and/or intrapulmonary shunt, as long as the measurement is performed in steady state. Steady state is a prerequisite for the Fick principle, as only under these circumstances does carbon dioxide exchange equal carbon dioxide production. As with every method of cardiac output measurement, the obtained value of cardiac output should be interpreted with knowledge of the actual hemodynamic circuit with the potential existence of shunts. Possible limitations of the mCO2F method are the potential calculation errors due to a too small venoarterial carbon dioxide concentration difference.
In this study, the lambs were ventilated via a cuffed endotracheal tube. However, newborns are always intubated with an uncuffed tube. One should be cautious in interpreting cardiac output values in the presence of an endotracheal tube leakage. A large leakage of the endotracheal tube leads to an underestimation of V˙CO2 and will result in a falsely lower cardiac output.
The mCO2F method of measuring cardiac output requires a steady state. The most interesting moments for cardiac output measurements are probably characterized by an absence of steady state. However, in our model, we were able to determine cardiac output reliably in hemodynamically unstable lambs, when defining steady state as steady carbon dioxide exchange for at least 6 min.
Sampling the RA for measuring the venous carbon dioxide concentration resulted in the most accurate calculated cardiac output. In general, in patients in a neonatal intensive care unit, the tip of a central venous catheter is positioned in the transition zone between the RA and SVC or IVC. However, this study shows that the position of the tip of the central venous catheter, in the IVC or SVC, has no significant influence on the measurement of cardiac output with the mCO2F method.
In this study, QmCO2F underestimated pulmonary blood flow. Although statistically significant, the bias is small and of no clinical relevance.
An evaluation of the mCO2F method according to the eight desirable characteristics for cardiac output monitoring described by Shephard et al. (18) shows that the accuracy and reproducibility are adequate and that the method is easy to apply and operator independent.
Several methods for cardiac output measurement in children are available, such as thermodilution, oxygen Fick and dye indicator techniques, bioimpedance, and Doppler echocardiography. These methods differ in applicability due to technical and size constraints and reliability (1–3). In comparison with published data concerning bias and precision of several methods of cardiac output measurement in animals (19–22), children (23–30), and adults (31–38), this study produced favorable results.
Without the use of additional equipment, one can measure cardiac output by the mCO2F method. It requires gas analysis of both arterial and central venous blood as well as of expiratory air by in-line capnography and simultaneous measurement of expiratory airflow. Usually, this is feasible in critically ill patients in a neonatal intensive care. This suggests that the method may be cost-effective without a risk of additional morbidity or mortality. Unfortunately, the response time of this method is not very fast because steady state is a prerequisite. This also implies a semicontinuous method of cardiac output monitoring.
We conclude that measurement of QmCO2F method is reliable in newborn lambs without a ductal shunt and can be used for trend monitoring. Venous blood sampling from the RA, SVC, or IVC can be used for this measurement. The mCO2F method is a promising technique that can be used to measure cardiac output in critically ill newborns.
Abbreviations
- IVC:
-
inferior vena cava
- mCO2F:
-
modified carbon dioxide Fick
- QmCO2F:
-
mCO2F method–derived cardiac output
- Qufp:
-
cardiac output determined by pulmonary ultrasonic flow probe
- RA:
-
right atrium
- SVC:
-
superior vena cava
- V˙CO2:
-
pulmonary carbon dioxide exchange
References
Fanconi S, Burger R 1992 Measurement of cardiac output in children. Intensive Care World 9: 8–12
Tibby SM, Murdoch IA 2002 Measurement of cardiac output and tissue perfusion. Curr Opin Pediatr 14: 303–309
Tibby SM, Murdoch IA 2003 Monitoring cardiac function in intensive care. Arch Dis Child 88: 46–52
Tibby SM, Hatherill M, Marsh MJ, Murdoch IA 1997 Clinicians' abilities to estimate cardiac index in ventilated children and infants. Arch Dis Child 77: 516–518
Bayliss J, Norell M, Ryan A, Thurston M, Sutton GC 1983 Bedside haemodynamic monitoring: experience in a general hospital. BMJ (Clin Res Ed) 287: 187–190
Henderson LJ, Bock AV, Field HJ, Stoddard JL 1924 Blood as a physicochemical system II. J Biol Chem 59: 379–431
Kelman GR 1967 Digital computer procedure for the conversion of PCO2 into blood CO2 content. Respir Physiol 3: 111–115
Godfrey S 1970 Manipulation of the indirect Fick principle by a digital computer program for the calculation of exercise physiology results. Respiration 27: 513–532
McHardy GJ 1967 The relationship between the differences in pressure and content of carbon dioxide in arterial and venous blood. Clin Sci 32: 299–309
Douglas AR, Jones NL, Reed JW 1988 Calculation of whole blood CO2 content. J Appl Physiol 65: 473–477
Jaffe MB 1998 Dead space and carbon dioxide production—technical issues. 9801 Rev 00, 1-7 Novametrix Medical Systems Inc.
Bland JM, Altman DG 1986 Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1: 307–310
Fick A 1870 [On the measurement of the blood quantity in the ventricles of the heart]. [Proceedings of the Physiological, Medical Society of Wurzburg], July 9, 1870.
Greisen G 2005 Autoregulation of cerebral blood flow in newborn babies. Early Hum Dev 81: 423–428
Mahutte CK, Jaffe MB 1995 Effect of measurement errors on cardiac output calculated with O2 and modified CO2 Fick methods. J Clin Monit 11: 99–108
Mahutte CK, Jaffe MB, Sassoon CS, Wong DH 1991 Cardiac output from carbon dioxide production and arterial and venous oximetry. Crit Care Med 19: 1270–1277
Mahutte CK, Jaffe MB, Chen PA, Sasse SA, Wong DH, Sassoon CS 1994 Oxygen Fick and modified carbon dioxide Fick cardiac outputs. Crit Care Med 22: 86–95
Shephard JN, Brecker SJ, Evans TW 1994 Bedside assessment of myocardial performance in the critically ill. Intensive Care Med 20: 513–521
Marx G, Sumpelmann R, Schuerholz T, Thorns E, Heine J, Vangerow B, Rueckoldt H 2000 Cardiac output measurement by arterial thermodilution in piglets. Anesth Analg 90: 57–58
Ruperez M, Lopez-Herce J, Garcia C, Sanchez C, Garcia E, Vigil D 2004 Comparison between cardiac output measured by the pulmonary arterial thermodilution technique and that measured by the femoral arterial thermodilution technique in a pediatric animal model. Pediatr Cardiol 25: 119–123
Peyton PJ, Venkatesan Y, Hood SG, Junor P, May C 2006 Noninvasive, automated and continuous cardiac output monitoring by pulmonary capnodynamics: breath-by-breath comparison with ultrasonic flow probe. Anesthesiology 105: 72–80
Bajorat J, Hofmockel R, Vagts DA, Janda M, Pohl B, Beck C, Noeldge-Schomburg G 2006 Comparison of invasive and less-invasive techniques of cardiac output measurement under different haemodynamic conditions in a pig model. Eur J Anaesthesiol 23: 23–30
Murdoch IA, Marsh MJ, Tibby SM, McLuckie A 1995 Continuous haemodynamic monitoring in children: use of transoesophageal Doppler. Acta Paediatr 84: 761–764
McLuckie A, Murdoch IA, Marsh MJ, Anderson D 1996 A comparison of pulmonary and femoral artery thermodilution cardiac indices in paediatric intensive care patients. Acta Paediatr 85: 336–338
Tibby SM, Hatherill M, Marsh MJ, Morrison G, Anderson D, Murdoch IA 1997 Clinical validation of cardiac output measurements using femoral artery thermodilution with direct Fick in ventilated children and infants. Intensive Care Med 23: 987–991
Linton RA, Jonas MM, Tibby SM, Murdoch IA, O'Brien TK, Linton NW, Band DM 2000 Cardiac output measured by lithium dilution and transpulmonary thermodilution in patients in a paediatric intensive care unit. Intensive Care Med 26: 1507–1511
Pauli C, Fakler U, Genz T, Hennig M, Lorenz HP, Hess J 2002 Cardiac output determination in children: equivalence of the transpulmonary thermodilution method to the direct Fick principle. Intensive Care Med 28: 947–952
Chew MS, Poelaert J 2003 Accuracy and repeatability of pediatric cardiac output measurement using Doppler: 20-year review of the literature. Intensive Care Med 29: 1889–1894
Levy RJ, Chiavacci RM, Nicolson SC, Rome JJ, Lin RJ, Helfaer MA, Nadkarni VM 2004 An evaluation of a noninvasive cardiac output measurement using partial carbon dioxide rebreathing in children. Anesth Analg 99: 1642-1647 tabtable
Botte A, Leclerc F, Riou Y, Sadik A, Neve V, Rakza T, Richard A 2006 Evaluation of a noninvasive cardiac output monitor in mechanically ventilated children. Pediatr Crit Care Med 7: 231–236
de Abreu MG, Geiger S, Winkler T, Ragaller M, Pfeiffer T, Leutheuser D, Albrecht DM 2002 Evaluation of a new device for noninvasive measurement of nonshunted pulmonary capillary blood flow in patients with acute lung injury. Intensive Care Med 28: 318–323
Della Rocca G, Costa MG, Pompei L, Coccia C, Pietropaoli P 2002 Continuous and intermittent cardiac output measurement: pulmonary artery catheter versus aortic transpulmonary technique. Br J Anaesth 88: 350–356
Kroon M, Groeneveld AB, Smulders YM 2005 Cardiac output measurement by pulse dye densitometry: comparison with pulmonary artery thermodilution in post-cardiac surgery patients. J Clin Monit Comput 19: 395–399
Tan HL, Pinder M, Parsons R, Roberts B, van Heerden PV 2005 Clinical evaluation of USCOM ultrasonic cardiac output monitor in cardiac surgical patients in intensive care unit. Br J Anaesth 94: 287–291
Sharma J, Bhise M, Singh A, Mehta Y, Trehan N 2005 Hemodynamic measurements after cardiac surgery: transesophageal Doppler versus pulmonary artery catheter. J Cardiothorac Vasc Anesth 19: 746–750
Sander M, von Heymann C, Foer A, von Dossow V, Grosse J, Dushe S, Konertz WF, Spies CD 2005 Pulse contour analysis after normothermic cardiopulmonary bypass in cardiac surgery patients. Crit Care 9: R729–R734
Lafanechere A, Albaladejo P, Raux M, Geeraerts T, Bocquet R, Wernet A, Castier Y, Marty J 2006 Cardiac output measurement during infrarenal aortic surgery: echo-esophageal Doppler versus thermodilution catheter. J Cardiothorac Vasc Anesth 20: 26–30
Chand R, Mehta Y, Trehan N 2006 Cardiac output estimation with a new Doppler device after off-pump coronary artery bypass surgery. J Cardiothorac Vasc Anesth 20: 315–319
Acknowledgements
The authors thank T. Arts and A.E.J. Hanssen from the Animal Laboratory, and J.A.M. Evers from the Department of Physiology, Radboud University Nijmegen Medical Centre for their outstanding support during this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
There was no external financial assistance for this study.
Rights and permissions
About this article
Cite this article
de Boode, W., Hopman, J., Daniëls, O. et al. Cardiac Output Measurement Using a Modified Carbon Dioxide Fick Method: A Validation Study in Ventilated Lambs. Pediatr Res 61, 279–283 (2007). https://doi.org/10.1203/pdr.0b013e318030d0c6
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/pdr.0b013e318030d0c6