Abstract
Newborn resuscitation with pure oxygen may be associated with long-term detrimental effects. Due to the change in attitude toward use of less oxygen upon resuscitation, there is a need to study effects of intermediate hyperoxia. The aim was to study dose-response correlation between inspiratory fraction of oxygen used for resuscitation and urinary markers of oxidative damage to DNA and amino acids. Hypoxemia was induced in newborn piglets following a standardized model; they were resuscitated for 15 min with either 21%, 40%, 60% or 100% oxygen and observed for 1 h. Urine samples were collected. Urinary elimination of 8-hydroxy-2′-deoxyguanosine (8-oxo-dG), 2′deoxyguanosine (2dG), ortho-tyrosine (o-Tyr) and phenylalanine (Phe) were determined by HPLC and tandem mass spectrometry (HPLC-MS/MS). Quotient of 8-oxo-dG/2dG and o-Tyr/Phe ratios were significantly and dose-dependant higher in piglets resuscitated with supplementary oxygen. 8-oxodG/dG: Mean (SD) 5.76 (1.81) versus 22.44 (12.55) p < 0.01 and o-Tyr/Phe: 19.07 (10.7) versus 148.7 (59.8)for 21% versus 100%, p < 0.001. Hypoxia and subsequent resuscitation for 15 min with graded inspiratory fraction of oxygen causes increased oxidative stress and a dose-dependant oxidation of DNA and Phenylalanine. The increase in the hydroxyl attack may lead to a pro-oxidative status and risk for genetic instability.
Similar content being viewed by others
Main
The transition from fetal to neonatal life contains complex and rapid physiologic changes. Usually, these changes are spontaneous, but approximately 10% of newborn babies require some assistance to begin breathing, and about 1% requires extensive resuscitation (1).
Compared with the intrauterine pO2 at about 3.3 kPa every child comes out to a relative hyperoxia and the values will normally spontaneously rise to about 8kPa during the first 30 min pp. Excessive hyperoxia may disturb a tuned equilibrium in the oxidative-antioxidative system. Exposure to hyperoxia generates significant higher levels of free radicals, and even more when preceded by hypoxia (2).
In the neonatal literature an increasing number of studies focusing on supplementary oxygen shows how oxidative stress influences both mortality and morbidity (3,4). Moreover, babies exposed to high oxygen concentrations in the first minutes of postnatal life have been shown to be at higher risk of childhood leukemia and cancer (5,6) making it mandatory to search for the causal connection.
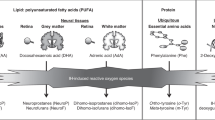
We wanted to explore possible reasons for long-term effects of oxidative stress as well as to search for signs of oxidative damage to DNA and proteins and have chosen two biomarkers of oxidative stress; 8-hydroxy-2′-deoxyguanosine (8oxodG) and ortho-tyrosine (o-Tyr). Urinary excretion of modified nucleosides/bases i.e., 8oxodG can be measured to assess oxidative damage on the level of the whole organism (7) and has been used to evaluate oxidative stress in patients (8). O-tyr is known to be formed entirely upon reaction of Phe with hydroxyl radicals and can also be measured in urine (9).
In addition, we also wanted to study the effect of re oxygenation with different oxygen concentrations to see if there is a dose-dependency or if there could be a threshold beyond which supplementary oxygen seems to be detrimental.
We hypothesized that resuscitation of piglets with supplementary oxygen (40%, 60% or 100%) would cause increased oxidative stress, more reactive oxygen species (ROS), and more damage to proteins and DNA than 21% oxygen.
MATERIALS AND METHODS
The National Animal Research Authority, (NARA), approved the experimental protocol. The animals were cared for and handled in accordance with the European Guidelines for Use of Experimental Animals.
Surgical preparation and anesthesia.
Thirty-eight newborn Noroc (LYxLD) pigs (20 male, 18 female) were included in this study, inclusion criteria being 12–36 h, Hb >5g/dL and good general condition. The piglets were anaesthetized, tracheotomized, ventilated and surgically prepared as previously described by Munkeby et al. (10).
Experimental Protocol.
The animals were stabilized for one hour after the initial procedures. Hypoxemia was induced by ventilation of 8% O2 in N2 and maintained until Base Excess (BE) reached -20 mmol/L or mean arterial blood pressure (MABP) decreased to 15 mm Hg. The saline infusion was stopped and CO2 was added during the whole duration of the hypoxemia aiming at a PaCO2 level of 8.0–9.5 kPa. Before start of resuscitation, piglets were randomized into four different groups. Thus, they were resuscitated for 15 min by ventilation with either 21% (n = 8), 40% (n = 9), 60% (n = 8) or 100% (n = 6) oxygen. Thereafter, they were observed for 60 min, ventilated with 21% oxygen, before receiving a lethal dose of pentobarbital (150 mg/kg iv). The abdominal wall was opened and the urine collected through direct aspiration from the visible bladder, and immediately snap frozen on liquid nitrogen and stored at minus 70°C. Laboratory personnel were blinded to the percentage oxygen administered, the study staff not.
Seven piglets, referred to as baseline pigs, were SHAM operated controls that went through surgery and 1 h of stabilization on ventilator and they were not exposed to hypoxia and reoxygenation (by drawing lots).
High performance liquid chromatography and mass spectrometry.
Determination of ortho-tyrosine (o-Tyr), phenylalanine (Phe), 8-hydroxy-2′-deoxyguanosine (8-oxodG) and 2′-deoxyguanosine (dG) in urine by High Performance Liquid Chromatography and Mass Spectrometry (HPLC-MS/MS).
HPLC-MS/MS was carried out using a Quattro Micro triple-quadruple mass spectrometer (Micromass, Manchester, UK) equipped with a Shimadzu LC-10ADvp. pump and a SLC-10Avp. Controller system with a SIL-10ADvp. auto injector. Samples were analyzed by reverse-phase HPLC using a Phenomenex (Torrance, CA) Prodigy ODS column (100 × 2 mm) with 3-μm particle size. In all cases 30 μL were injected onto the column. The temperature of the column was maintained at 25°C. The following gradient system, pumped through the column at 0.2 mL/min, was used (min/%A/%B) (A, 0.1% formic acid; B, methanol): 0/95/5, 10/95/5, 15/95/5, 15.1/95/5, 30/95/5. Positive ion electro spray tandem mass spectra were recorded with the electro spray capillary set at 3.5 keV and a source block temperature of 120°C. Nitrogen was used as the drying and nebulising gas at flow rates of 300 and 30 L/h, respectively. Argon at 1.5 × 10−3 mbar was used as the collision gas for collision-induced dissociation. An assay based on HPLC-MS/MS with multiple reaction monitoring was developed using the transitions m/z 182→136 for o-Tyr, 166→120 for Phe, 284→168 for 8-oxodG, and 268→152 for dG, all of which represent favorable fragmentation pathways for these protonated molecules. Calibration curves were obtained using a standard (0.001–10 μM) and were in each case found to be linear with correlation coefficients >0.99. The limits of detection and quantitation for our method were of 0.001 μM.
Statistics.
SPSS 13 for Windows® was used for the baseline characteristics of the piglets. Statistical analysis was performed in two steps. One-way analysis of variance (ANOVA) was performed first. When the overall comparison of groups was significant, differences between individual groups were investigated by Tukey's method. Differences were considered to be significant at p < 0.05. Nonparametric statistics (Mann Whitney's U test) for comparison of nonpaired samples were used for the HPLC-MS/MS results, as data did not show normal distribution.
RESULTS
The piglets had a mean (SD), weight of 1798 (±198) grams, Hb was 7.5 (±1.1) g/dL and the mean time of hypoxemia was 60.3 (±34.3) min. These variables were not statistically different between the groups. The arterial blood gases are provided in Table 1.
Both 8-oxodG and o-tyrosine showed a dose-dependant increase according to the oxygen concentration used upon resuscitation (Table 2). The values should be expressed as a ratio to the parental product, for 8-oxodG, dG and for o-Tyr, -Phe, (Fig. 1 and Fig. 2). Since the amount of dG and Phe is one order of magnitude greater, results are given with the following factor: o-Tyr (μmol/L)/Phe (μmol/L) × 100 and 8-oxodG/2 dG × 1000.
Quotient 8-hydroxy-2′-deoxyguanosine (μmol/L)/2′ deoxyguanosine(μmol/L ×103) ratio in asphyxiated piglets resuscitated with 21% O2 (Roomair) (n = 8) 5.76 (1.81) or various oxygen concentrations: FiO2 40% (n = 9) 8.44 (4.81); FiO2 60% (n = 8) 10.82 (5.55); FiO2 100% (n = 6) 22.44 (12.55). Values are expressed as mean (±SD) * p < 0.01.
Quotient O-tyrosine (μmol/L)/Phenylalanine (μmol/L × 102) in asphyxiated piglets resuscitated with 21% O2 (room air) (n = 8) 19.07 (10.7) or various oxygen concentrations): FiO2 40% (n = 9) 56.9 (18.9); FiO2 60% (n = 8) 87.7 (32.1); FiO2 100% (n = 6) 148.7 (59.8). Values are expressed as mean (±SD) ** p < 0.01, * p < 0.05; vs Control 7.58 (2.33) † p < 0.001 vs 21%.
The quotients of 8-oxodG/2dG as well as o-Tyr/Phe ratios were significantly higher in piglets resuscitated with either 40%, 60% or 100% oxygen compared with room air, (p < 0.01for the 8-oxodG/dG and p < 0.001 for o-tyr/Phe).
DISCUSSION
The generation of reactive oxygen species may be both beneficial and detrimental to cells. ROS have a function in inter- and intracellular signaling but they have been associated with numerous diseases due to their capability to modify cellular bio-molecules (11,12). If there comes to an imbalance between the ROS-producing, pro-oxidant factors, and the antioxidant defense, ROS can cause damage to lipids, proteins and DNA. Modified lipids and proteins may be removed via normal turnover of molecules, but damage to DNA has to be repaired. To maintain the DNA integrity the removal of DNA lesions by cellular repair processes is crucial to limit mutagenesis, cytostasis and cytotoxicity (12).
Among oxygen radicals, the hydroxyl radical ·OH is by far the most relevant to patho-physiology (13). Since the half-life time of ·OH is extremely short (10−9 s), it cannot be measured directly. But stable end products of oxidative damage to proteins and DNA that are excreted in the urine may be used (14). Guanine is the DNA base most prone to oxidation. The most studied oxidative DNA lesion product is 8-hydroxyguanine (8-oxo-Gua) along with its 2′-deoxynucleoside equivalent, 7,8-dihydro-8-oxo-2′-deoxyguanosine (8-oxodG). 8-oxo-Gua is one of the most mutagenic lesions so far discovered and must be removed prior to DNA replication. The oxidized base, 8-oxodG, is excreted unchanged in the urine and may be assessed as the oxidative damage on the level of the whole organism. Urinary levels of 8-oxodG are not influenced by diet, cell death or artefactual formation, and may serve as a good biomarker for oxidative stress to DNA (15,16). The levels of urinary 8-oxodG in different species appear to correlate well with species-specific metabolic rates (7).
O-tyrosine is formed entirely upon reaction of the essential amino acid phenylalanine with hydroxyl radicals and is therefore known to be a specific marker of hydroxyl radical attack. It has been strongly associated with oxygen treatment in neonates (9).
We found o-Tyr and 8-oxodG to represent good and reliable markers for ·OH detection. Both o-Tyr and 8-oxodG remains stable upon storage and deep-frozen for several months (17,18). They can be obtained in a noninvasive way and may be reproduced as necessary in the experimental setting. In addition, in the clinical setting, they can be used without having to perform invasive procedures. Both parameters reflect ROS' chemically reaction with nearby molecules and their rise in urine is almost inherent to the process of oxidative stress.
The creatinine concentration may not be constant in the asphyxiated piglets urine, so the concentrations were instead expressed as ratios over the parental amino acids for each oxidized product; o-tyr/Phe and 8-oxodG/dG (17,19).
HPLC-MS/MS is a powerful technology that can overcome the sensitivity and selectivity issues in analysis of DNA adducts and oxidized amino acids.
It requires only small volumes of urine and no pretreatment or prepurification steps. It allows a noninvasive measurement of oxidative stress in vivo and enables repeated monitoring under disease conditions (20). It has been used for the measurement of oxidative stress in preterm neonates urine (21), and in a big population study aiming to clarify if oxidative damage to DNA may be important in carcinogenesis and a possible risk factor for lung cancer (22).
The oxidation of guanine in DNA is not the only source of the urinary 8-oxodG. In the study of Renner et al. 2000, they found a high intra-individual and inter-individual variation in the urinary excretion of 8-oxodG (70%) indicating that additional factors may influence the formation and excretion of 8-oxodG (19). That may be one reason for the relatively high values we found in our Control-group. The metabolic rate will influence the 8-oxodG formation and the nonasphyxiated and non fluid restricted piglets in the Control group could therefore have a higher background activity (23). Within each group, the piglets will have an inter-individual genetic diversity comparable to newborn babies and distinct differing from transgenic modified animals.
There are some limitations of the study. ROS (and most notably ·OH) generate a large number of modifications in DNA by a variety of mechanisms. Measurement of a single product like 8-oxodG thereby just represents a part of the picture (24). Our control group serves as a possibility to explore how the different values would be in equal surgical prepared anesthetized animals not going through hypoxia and reoxygenation. However, they may not serve as a proper control group for the study of urinary 8oxodG because they had other conditions for regulating metabolic rate and their life span was shorter.
We also considered trying to get a urine sample before start of the trial, but nearly all the piglets had a spontaneous miction just by arrival at our research unit.
This study had a short duration and one should be cautious to draw conclusions for long-term effects. We are planning to do a follow up study with urine measurements after a 9-h observation time after exposure to hyperoxia. However earlier studies (25), have shown a persistence of oxidative stress as long as 4 wk in newborn babies exposed to a short duration of 100% oxygen.
An animal model for human newborn resuscitation will always just be an approximation to the real clinical situation. The lung maturation in piglets at birth is advanced compared with human newborns. The airways and associated blood vessels are completely formed at birth, whereas parenchymal development occurs primarily after birth (26). In contrast to the human newborn the chest wall of the newborn piglet is stiff (27). But before 3 d of age the piglet may be an appropriate model for human brain development, also with regard to the patterns of cellular development and myelination (28).
Since this is an animal study, we do not have the large groups one could prefer due to resources and the aim of limit the use of laboratory animals.
The strengths of the study is standardization of the hypoxia-reoxygenation episodes, equal handling of the urine, the specific determination of 8oxodG and o-Tyr through the HPLC-MS/MS technique, and that we are looking at the differences between the equally treated randomized groups.
To our knowledge there have been no studies measuring 8-oxodG and o-Tyrosine shortly after hypoxia and reoxygenation with supplementary oxygen. We found the distinct dose-dependant increase in the 40, 60 and 100% group rise concerns. Both o-Tyr and 8-oxodG can be easily determined in urine and may be used in a clinical setting. The o-Tyr/Phe ratio is especially sensitive and specific.
CONCLUSION
Our findings of significant increase in both urinary o-tyrosine and 8-oxodG levels after 15 min of hyperoxia by resuscitation strongly indicates that using 40, 60 or 100% oxygen by resuscitation gives increased oxidative stress and a dose dependent increase in the experienced hydroxyl attack. It may lead to a pro-oxidative status and risk of genetic instability.
Our findings support the hypothesis that using 100% oxygen, or even 60 or 40%, for resuscitation after global hypoxia is potentially harmful compared to room air.
Abbreviations
- 8-oxodG:
-
8-hydroxy-2′-deoxyguanosine
- dG:
-
2′deoxyguanosine
- HPLC-MS/MS:
-
high-performance liquid chromatography and tandem mass spectrometry
- o-Tyr:
-
ortho-tyrosine
- Phe:
-
phenylalanine
- ROS:
-
reactive oxygen species
References
International Liaison Committee on Resuscitation 2005 2005 Committee on Resuscitation 2005 2005 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Part 7: Neonatal resuscitation. Resuscitation 67: 293–303.
Kondo M, Itoh S, Isobe K, Kondo M, Kunikata T, Imai T, Onishi S 2000 Chemiluminescence because of the production of reactive oxygen species in the lungs of newborn piglets during resuscitation periods after asphyxiation load. Pediatr Res 47: 524–527
Saugstad OD, Ramji S, Vento M 2005 Resuscitation of depressed newborn infants with ambient air or pure oxygen: a meta-analysis. Biol Neonate 87: 27–34
Vento M, Sastre J, Asensi MA, Vina J 2005 Room-air resuscitation causes less damage to heart and kidney than 100% oxygen. Am J Respir Crit Care Med 172: 1393–1398
Naumburg E, Bellocco R, Cnattingius S, Jonzon A, Ekbom A 2002 Supplementary oxygen and risk of childhood lymphatic leukaemia. Acta Paediatr 91: 1328–1333
Spector LG, Klebanoff MA, Feusner JH, Georgieff MK, Ross JA 2005 Childhood cancer following neonatal oxygen supplementation. J Pediatr 147: 27–31
Shigenaga MK, Gimeno CJ, Ames BN 1989 Urinary 8-hydroxy-2′-deoxyguanosine as a biological marker of in vivo oxidative DNA damage. Proc Natl Acad Sci USA 86: 9697–9701
Wu LL, Chiou CC, Chang PY, Wu JT 2004 Urinary 8-OHdG: a marker of oxidative stress to DNA and a risk factor for cancer, atherosclerosis and diabetics. Clin Chim Acta 339: 1–9
Lubec G, Widness JA, Hayde M, Menzel D, Pollak A 1997 Hydroxyl radical generation in oxygen-treated infants. Pediatrics 100: 700–704
Munkeby BH, Borke WB, Bjornland K, Sikkeland LI, Borge GI, Halvorsen B, Saugstad OD 2004 Resuscitation with 100% O2 increases cerebral injury in hypoxemic piglets. Pediatr Res 56: 783–790
Cooke MS, Olinski R, Evans MD 2006 Does measurement of oxidative damage to DNA have clinical significance?. Clin Chim Acta 365: 30–49
Evans MD, Dizdaroglu M, Cooke MS 2004 Oxidative DNA damage and disease: induction, repair and significance. Mutat Res 567: 1–61
Biondi R, Ambrosio G, Liebgott T, Cardounel AJ, Bettini M, Tritto I, Zweier JL 2006 Hydroxylation of D-phenylalanine as a novel approach to detect hydroxyl radicals: application to cardiac pathophysiology. Cardiovasc Res 71: 322–330
de Zwart LL, Meerman JH, Commandeur JN, Vermeulen NP 1999 Biomarkers of free radical damage applications in experimental animals and in humans. Free Radic Biol Med 26: 202–226
Cooke MS, Evans MD, Dove R, Rozalski R, Gackowski D, Siomek A, Lunec J, Olinski R 2005 DNA repair is responsible for the presence of oxidatively damaged DNA lesions in urine. Mutat Res 574: 58–66
Olinski R, Rozalski R, Gackowski D, Foksinski M, Siomek A, Cooke MS 2006 Urinary measurement of 8-OxodG, 8-OxoGua, and 5HMUra: a noninvasive assessment of oxidative damage to DNA. Antioxid Redox Signal 8: 1011–1019
Orhan H, Vermeulen NP, Tump C, Zappey H, Meerman JH 2004 Simultaneous determination of tyrosine, phenylalanine and deoxyguanosine oxidation products by liquid chromatography-tandem mass spectrometry as non-invasive biomarkers for oxidative damage. J Chromatogr B Analyt Technol Biomed Life Sci 799: 245–254
Cooke MS, Lunec J, Evans MD 2002 Progress in the analysis of urinary oxidative DNA damage. Free Radic Biol Med 33: 1601–1614
Renner T, Fechner T, Scherer G 2000 Fast quantification of the urinary marker of oxidative stress 8-hydroxy-2′-deoxyguanosine using solid-phase extraction and high-performance liquid chromatography with triple-stage quadrupole mass detection. J Chromatogr B Biomed Sci Appl 738: 311–317
Tsukahara H, Jiang MZ, Ohta N, Sato S, Tamura S, Hiraoka M, Maeda M, Mayumi M 2004 Oxidative stress in neonates: evaluation using specific biomarkers. Life Sci 75: 933–938
Tsukahara H, Toyo-Oka M, Kanaya Y, Ogura K, Kawatani M, Hata A, Hiraoka M, Mayumi M 2005 Quantitation of glutathione S transferase-pi in the urine of preterm neonates. Pediatr Int 47: 528–531
Loft S, Svoboda P, Kasai H, Tjonneland A, Vogel U, Moller P, Overvad K, Raaschou-Nielsen O 2006 Prospective study of 8-oxo-7,8-dihydro-2′-deoxyguanosine excretion and the risk of lung cancer. Carcinogenesis 27: 1245–1250
Greenberg JA, Wei H, Ward K, Boozer CN 2000 Whole-body metabolic rate appears to determine the rate of DNA oxidative damage and glycation involved in aging. Mech Ageing Dev 115: 107–117
Dizdaroglu M, Jaruga P, Birincioglu M, Rodriguez H 2002 Free radical-induced damage to DNA: mechanisms and measurement. Free Radic Biol Med 32: 1102–1115
Vento M, Asensi M, Sastre J, Garcia-Sala F, Pallardo FV, Vina J 2001 Resuscitation with room air instead of 100% oxygen prevents oxidative stress in moderately asphyxiated term neonates. Pediatrics 107: 642–647
Mansell AL, McAteer AL, Pipkin AC 1992 Maturation of interdependence between extra-alveolar arteries and lung parenchyma in piglets. Circ Res 71: 701–710
Standaert TA, Wilham BE, Mayock DE, Watchko JF, Gibson RL, Woodrum DE 1991 Respiratory mechanics of the piglet during the first month of life. Pediatr Pulmonol 11: 294–301
Pond WG, Boleman SL, Fiorotto ML, Ho H, Knabe DA, Mersmann HJ, Savell JW, Su DR 2000 Perinatal ontogeny of brain growth in the domestic pig. Proc Soc Exp Biol Med 223: 102–108
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by Helse Sor.
Rights and permissions
About this article
Cite this article
Solberg, R., Andresen, J., Escrig, R. et al. Resuscitation of Hypoxic Newborn Piglets With Oxygen Induces a Dose-Dependent Increase in Markers of Oxidation. Pediatr Res 62, 559–563 (2007). https://doi.org/10.1203/PDR.0b013e318156e8aa
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/PDR.0b013e318156e8aa
This article is cited by
-
Splanchnic oxygen saturation during reoxygenation with 21% or 100% O2 in newborn piglets
Pediatric Research (2022)
-
Randomized trial of oxygen weaning strategies following chest compressions during neonatal resuscitation
Pediatric Research (2021)
-
Free radicals and neonatal encephalopathy: mechanisms of injury, biomarkers, and antioxidant treatment perspectives
Pediatric Research (2020)
-
Oxygen therapy of the newborn from molecular understanding to clinical practice
Pediatric Research (2019)
-
Increased expression of inflammatory genes in the neonatal mouse brain after hyperoxic reoxygenation
Pediatric Research (2015)