Abstract
Hypoxic ischemic (HI) injury in neonates may have devastating, long-term consequences. Recently completed clinical trials in HI neonates indicate that hypothermia within 6 h of birth results in modest improvement in the combined outcome of death or severe disability. The aim of this study was to investigate the effects of combining hypothermia and N-acetylcysteine (NAC) on brain injury, neonatal reflexes and myelination after neonatal HI. Seven-day-old rats were subjected to right common carotid artery ligation and hypoxia (8% oxygen) for 2 h. Systemic hypothermia (30 + 0.5°C) was induced immediately after the period of HI and was maintained for 2 h. NAC (50 mg/kg) was administered by intraperitoneal injection daily until sacrifice. Brain infarct volumes were significantly reduced at 48 h post-HI in the hypothermia plus NAC group (21.5 ± 3.84 mm3) compared with vehicle (240.85 ± 4.08 mm3). Neonatal reflexes were also significantly improved by combination therapy at days 1 and 7. There was a significant loss of right hemispheric brain volume in the untreated group at 2 and 4 wk after HI insult. Brain volumes were preserved in hypothermia plus NAC group and were not significantly different when compared with the sham group. Similarly, increased myelin expression was seen in brain sections from hypothermia plus NAC group, when stained for Luxol Fast Blue (LFB), Myelin Basic Protein (MBP) and Proteolipid protein (PLP). These results indicate that hypothermia plus NAC combination therapy improves infarct volume, myelin expression and functional outcomes after focal HI injury.
Similar content being viewed by others
Main
Hypoxic ischemic (HI) injury in neonates occurs in term neonates at a frequency of 1–4 per 1,000 live births (1) and may result in reduced potential for motor and cognitive development (2,3). Hypothermia improves survival and neurologic outcomes in neonatal animal models of severe HI injury (4–7), and in recently completed clinical trials of HI neonates when instituted within 6 h of birth (8–11). Animal data shows that initiation of hypothermia between 6 and 12 h after HI reduces cerebral injury (12,13), perhaps moderating secondary injury cascades during a time when cellular recovery is still possible. However, some inflammatory mediators are merely delayed by hypothermia treatment (14), indicating that hypothermia may be most helpful in extending the therapeutic window after HI injury.
Therefore, the combination of hypothermia with other pharmacologic interventions may allow for significant improvements in outcome, which are not possible when the therapies are used separately. There have been limited reports of hypothermia treatment with other pharmacologic interventions. Systemic hypothermia and a pan-caspase inhibitor, boc-aspartyl-(OMe)-fluoromethyl-ketone (BAF), produced a strong protective effect against neuronal cell damage in the ipsilateral hippocampal CA1 region of the developing rat brain, along with a reduction in caspase-3 activity (15). The combination of MK-801 and hypothermia also protected animals against HI injury (16). These studies suggest that inhibiting some excitotoxic or inflammatory processes with combination therapy may have an agonistic effect on overall outcomes.
N-acetylcysteine (NAC) has been shown to scavenge oxygen free radicals, restore intracellular glutathione levels, improve cellular redox potential and reduce apoptosis. NAC attenuates reperfusion injury and decreases inflammatory cytokines and inducible nitric oxide synthase in an adult rat stroke model (17,18). NAC is an FDA-approved drug with an extensive safety profile in human neonates in the IV (intravenous) form.
In this report, we investigated the effect of N-acetylcysteine (NAC) and systemic hypothermia in a neonatal rat model of carotid artery ligation. Our data indicate that the combination of NAC and hypothermia improves infarct volume, myelin expression, and functional outcomes after focal HI injury in PND 7 rats.
MATERIALS AND METHODS
Animals.
Postnatal seven-day-old (PND 7) Sprague Dawley rats were used for the study (Harlan, Indianapolis, IN). After HI procedures, animals were separated from dams, kept under constant conditions and given standard chow, water ad libitum and kept in a 12/12 h light/dark cycle in the animal facility. All procedures were in accordance with the Guide for the Care and Use of Laboratory Animals adopted by the National Institutes of Health (USA) and approved by the Institutional Animal Care Committee.
Reagents.
Anti-Proteolipid protein (PLP; clone plpc 1; MCA839G) and anti-mouse myelin basic protein (MBP; clone 1 (129–138); MCA70) primary antibodies (Serotec, Raleigh, NC); 2, 3, 5-triphenyl-tetrazolium chloride (TTC) and Hoechst - 33258 dye (Sigma Chemical Co., St. Louis, MO); secondary antibodies of Texas red-conjugated goat anti-mouse IgG for MBP and fluorescein-conjugated goat anti-mouse IgG for PLP (Vector Laboratories, Burlingame, CA); 10% Neutral buffered formalin (Fisher Chemicals, Fairlawn, NJ); Novaplus™ (Isoflurane, USP) (Abbot Laboratories, North Chicago, IL).
Study design.
The protocol is shown in Fig. 1. PND 7 rats (n = 48) were randomly divided into groups: Sham operated animals did not undergo HI (Sham; n = 8); Vehicle treated animals (VEH; n = 10) were subjected to HI with no treatment; Hypothermia (Hypo; n = 10) underwent systemic hypothermia of 30 + 0.5°C, for 2 h; NAC treated animals (NAC; n = 10) were injected with 50 mg/kg NAC intraperitoneally (IP) once daily until sacrifice; and Hypothermia + NAC (HYP + NAC; n = 10) were treated with the combination of systemic hypothermia of 30 + 0.5°C, for 2 h and NAC 50 mg/kg IP once daily until sacrifice. A NAC dose of 50 m/kg was chosen from experiments in an adult rat stroke model which showed efficacy at 50-150 mg/kg/d and toxicity at 500 mg/kg/d (18). We chose repeated administration of NAC, as previous experiments in PND 7 rats revealed little neuropreservation at 48 h with a single dose of NAC, either alone or in combination with hypothermia (unpublished data).
Hypoxia-ischemia.
We used the modified Levine rat model of hypoxic ischemic injury (19,20) with unilateral ligation of right common carotid artery and exposure to a 8% oxygen atmosphere. Under isoflurane anesthesia, the right common carotid artery was identified through a longitudinal neck incision, isolated from the nerve and vein, and permanently occluded with silk suture (6-0). Animals were allowed to recover for 15 min under a heating lamp and returned to their dams. After 2 h of resting period, pups were placed into a Perspex chamber (immersed in a water bath) and exposed to warm humidified 8%/92% oxygen/nitrogen atmosphere (pre-mixed certified medical grade gas mixture; LifeGas, Norcross, GA) for 2 h, with the chamber temperature maintained at constant temperature of 37 + 0.5°C. A needle thermocouple temperature probe (Harvard Apparatus, Holliston, MA), was placed in the chamber to monitor the skin temperature of the pups/chamber thus provide constant thermal environment.
The pups were then administered NAC or hypothermia (30 + 0.5°C for 2 h), either alone or in combination. For systemic hypothermia the pups were then transferred into a Perspex chamber (immersed in a water bath) and exposed to normal cool air (certified medical grade air; LifeGas, Norcross, GA), with the chamber maintained at constant temperature of 30 + 0.5°C. A needle thermocouple temperature probe (Harvard Apparatus, Holliston, MA) connected to a heating pad was placed in the chamber to monitor the skin temperature of the pups' chamber thus providing constant thermal environment. Pups were then returned to their dams until weaning. Animals were weaned at 3 wk of age, given standard chow and water ad libitum, and kept in a 12/12 h light/dark cycle. All animals survived until sacrifice.
Independent effects of NAC on rectal temperature.
To assess the effects of NAC and HYP +NAC on core body temperature, a separate set of experiments was performed. All five groups (n = 2 sham, n = 6 each HI group) were treated per protocol, and rectal temperatures were measured every hour for 3 h after treatment in pups kept separate from dams. For the hypothermia groups, temperature was measured for the 3 h following return to ambient temperatures.
Neonatal reflexes.
Pups were assessed at 24 h and 1 wk after the HI insult, by negative geotaxis and cliff aversion tests for evidence of neurobehavioral impairment. For the geotaxis test, pups were placed head down on an inclined board (45°), with the hind limbs in the middle of the board. The time taken to turn around and climb up > 90° angle was recorded. For cliff aversion test, the rat snout and forepaws are placed over a cliff 60 cm high, and the time taken to withdraw and turn more than 45° from the edge of a flat surface was recorded. For both experiments, any animal failing to perform the test in the maximum allowed time of 20 s was assigned a time of 20 s. Three recordings per event were taken for each test.
Measurement of ischemic infarct at 48 hours.
Infarct volume was evaluated as previously described (19). After 48 h of reperfusion, the brains were quickly removed, placed in ice-cold saline for 5 min, and cut at 2-mm intervals from the frontal pole into 6 coronal sections. The slices were incubated in 2% 2-, 3-, 5-triphenyl-tetrazolium chloride (TTC, dissolved in saline) for 15 min at 37°C, fixed by immersion in 10% formalin, and images acquired in Photoshop 7.0 (Adobe Systems). The white areas of infarct were quantified using image-analysis software (Scion Corporation). The mean infarct volume per slice was obtained from the product of the average thickness of a slice (2 mm) and the area of infarct in that section. The volumes of each of the six sections were summed to give the total infarct area of the brain.
Measurement of right brain hemisphere volume.
Animals were killed at 2 or 4 wk and the right brain hemispheric volumes were measured (n = 3 each group). Brains were quickly removed, fixed by immersion in 10% formalin for 1 wk, cut into six coronal sections. Images of unstained sections of neonatal rat brain were acquired as detailed above. The mean volume of right hemisphere per slice was obtained from the product of the average thickness of a slice (2 mm) and the area of right hemisphere in that section. The volumes of each of the six sections were summed to give the total infarct area of the brain.
Immunohistochemistry.
For histochemical analysis, the brain tissue sections were fixed in 10%, embedded in paraffin and sectioned at 5 μm thickness. Sections were stained with luxol fast blue (LFB) and observed under light microscopy.
Staining for Myelin Basic protein (MBP) and Proteolipid protein (PLP) was carried out as described earlier (21). Briefly, paraffin-embedded, rat brain tissue sections were de-paraffinized and hydrated with xylene followed by graded alcohol. Sections were incubated overnight at 4°C with a 1:200 dilution of either anti-MBP antibody, or anti-PLP antibody. After the tissues were washed in PBS, staining was detected with 1:200 dilution of Texas-red conjugated goat anti-mouse IgG for MBP or fluorescein isothiocyanate (FITC)-conjugated Fluorescein conjugated anti-mouse IgG secondary antibody for PLP. Sections were also incubated with Texas red-conjugated IgG and fluorescein isothiocyanate (FITC)-conjugated IgG without primary antibody as a negative control. The slides were mounted using an aqueous mounting media containing Hoechst dye (Vectashield; Vector Laboratories, Burlingame, CA).
Statistical analysis.
All values are expressed as mean ± SD of n determinations obtained as indicated. Differences in means between the five groups were analyzed for significance using ANOVA, with repeated measures analysis and Bonferroni corrections where appropriate. Due to problems with ascertainment of normal distribution in small sample sizes, data were also analyzed with non-parametric tests (Kruskal-Wallis test) which gave identical results for statistical significance.
RESULTS
Combination treatment decreases infarct area at 48 hours.
The areas of infarct, noted by the white region in Fig. 2A with volumes quantified in Fig. 2B, are improved by all treatments compared with vehicle treated animals (mean total infarct volume VEH 240.85 ± 4.08 mm3; group ANOVA, p < 0.0001). NAC or Hypothermia treatment alone reduced mean infarct volume to 133.33 ± 5.01 mm3 and 107.52 ± 3.84 mm3, respectively. The combination of HYP + NAC at start of normoxia (0 h) significantly and dramatically reduced infarct volume at 48 h (21.5 ± 3.84 mm3), and showed greater reduction of the HI-induced infarct at 48 h than either treatment alone.
Cerebral infarction following HI in PND 7 rats. TTC staining was performed 48 h after HI insult. (A) Representative, consecutive, coronal brain sections from cranial to caudal regions are shown for each group. Lack of staining in the ipsilateral hemisphere of the vehicle group represents infarction (white area), which is attenuated by treatment. (B) Total Infarct volumes of ischemic right cerebral hemisphere by treatment group are presented as mean ± SD (n = 3). * HYP + NAC treated brains show reduced infarct volumes, significantly different from all other groups.
Hypothermia plus NAC treatment preserves brain volumes at two and four weeks.
HI neonatal rat brain sections show atrophy at 2 wk post-HI (VEH), but HYP + NAC treatment resulted in significant preservation of brain volume (Fig. 3A,B). The mean right hemispheric brain volume for rats treated with HYP + NAC (400.62 ± 29.95 mm3) was significantly greater than VEH (244.97 ± 6.25 mm3), Hypo (262.71 ± 33.03 mm3) groups, and NAC alone (323.93 ± 25.28 mm3). Neuropreservation with HYP + NAC was not significantly different from Sham controls (415.74 ± 12.87 mm3) at 2 wk (group ANOVA, p < 0.0001).
Neuroprotective effects of combination therapy on brain injury following HI insult. Representative unstained brains from each group are shown at 2 wk (A) or 4 wk (C) after HI insult. Right brain hemispheres were severely atrophic following HI insult in VEH group, but largely preserved in the HYP + NAC group. Mean total volumes of the right cerebral hemisphere in the 2 wk (B) and the 4 wk groups (D) are presented as mean ± SD (n = 3 each group). For both 2 and 4 wk time points (B and D; p < 0.05): * HYP + NAC were not significantly different from Sham; † NAC, Hypo and VEH were significantly different from Sham.
At 4 wk mean right brain hemisphere volume in the VEH group decreased to 229.27 ± 6.32 mm3 (Fig. 3D). HYP + NAC treatment significantly improved the right hemisphere brain volumes (404.69 ± 57.57 mm3) over Hypo alone (249.20 ± 21.38 mm3) and VEH groups, and was not significantly different from Sham 401.49 ± 32.30 mm3 (group ANOVA, p < 0.0003). Using Bonferroni corrections for multiple comparisons, HYP + NAC and NAC alone (322.22 ± 39.64 mm3) were not significantly different. Right hemispheric brain volumes in HYP + NAC group were 76% greater than vehicle, while NAC and hypothermia alone were 41% and 9% greater than vehicle, respectively. The combination of systemic hypothermia and NAC significantly decrease brain atrophy and appear to act synergistically in preserving right hemispheric volumes at 2–4 wk.
Improved functional outcomes with combination therapy.
We compared short-term neurobehavioral development between normal and HI PND 7 rats by cliff aversion and negative geotaxis on day 1 and 7 post-HI insult (group ANOVAs, p < 0.0004) (22,23). Both functional outcomes were significantly better in the HYP + NAC group compared with vehicle, NAC or hypothermia treatment alone at day 7 (Fig. 4A,B). Specifically, times in Geotaxis and Cliff aversion reflexes tests were reduced 62% and 52% respectively, at 7 d post-HI in the HYP + NAC compared with the VEH groups.
Combination treatment enhances the performances of negative geotaxis and cliff aversion tests compared with untreated HI animals. (A) Geotaxis reflexes were observed at 24 h (▪) and 7 d (□) after HI insult. VEH pups exhibited longer mean latency times (in seconds) compared with Sham and treatment groups. (n = 3 Sham, n = 5 all HI groups). The best time out of three recordings per animal was recorded, and the mean ± SD of each group are presented. For both A and B at the 7 d time point (p < 0.05): * HYP + NAC is not significantly different from Sham; † HYP + NAC is significantly different from Hypo, NAC alone.
Hypothermia plus NAC treatment improves myelination after HI insult in rat brains. Luxol-fast-blue (LFB) staining identifies phospholipids in the myelin (Fig. 5). Figs. 6 and 7 show Hoechst stained (blue fluorescence) corpus callosum and cingulum in 2 and 4 wk post-HI rats.
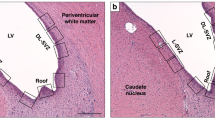
Combination treatment preserves myelination. Representative photomicrographs demonstrating relative decrease in LFB staining at 2 wk (A) and 4 wk (B) after HI insult in VEH rat brains compared with Sham, and preservation of LFB staining in HYP +NAC compared with the other treatment groups. Sections of rat brains for each group are from cingulum (A and B, sections 1-5) and corpus callosum (A and B, sections 6-10). (C) Representative H and E stained cross-sections of whole brain for each group at 4 wk show gross anatomical details of areas stained for histochemistry in Figs. 5 and 7 (A,B). The staining was performed as described on three different sections from two different rats. Magnification ×400.
Combination treatment preserves myelin expression 2 wk after HI injury. Representative photomicrographs of Hoechst staining & immunostaining for PLP (Fig. 6A, green fluorescence) and MBP (Fig. 6B, red fluorescence). Decreased staining for both markers is evident 2 wk after HI insult in the corpus callosum and cingulum in the VEH group. In contrast, HYP + NAC and Sham groups are not appreciably different in PLP or MBP staining, indicating preservation of myelin expression. Hoechst staining (blue fluorescence) did not show differences in cell number between the groups. The staining was performed as described on three different sections from two different rats. Magnification ×400.
Combination treatment attenuates reduced myelin expression 4 wk after HI insult. Representative photomicrographs of Hoechst staining with immunostaining for PLP (Fig. 7A, green fluorescence) and MBP (Fig. 7B, red fluorescence). In both A and B: decreased myelination in the corpus callosum and cingulum is evident 4 wk after HI insult in rat brain sections in the VEH group. HYP + NAC preserves myelination compared with the VEH group in all sections. HYP + NAC and Sham groups are not appreciably different in PLP and MBP staining. Hoechst staining (blue fluorescence) did not show differences in cell number between the groups. The staining was performed as described on three different sections from two different rats. Magnification ×400.
At 2 wk, LFB staining in the corpus callosum and cingulum shows a marked improvement in the expression of positive myelin sheaths in HYP + NAC, compared with VEH, NAC, or Hypo alone (Fig. 5A). Similar results were observed at 4 wk after HI insult (Fig. 5B). These results were further confirmed by the PLP and MBP staining for the protein components of myelin sheaths, as seen at 2 wk after HI insult, Fig. 6A (PLP, green florescence) and Fig. 6B (MBP, red florescence), and 4 wk after HI insult (Fig. 7A,B). There was no overall decrease in total cell numbers between groups by cell counts (ANOVA, p = 1.0), but there were significant differences in myelination among the treatment groups.
NAC had no effect on rectal temperature.
Comparing rectal temperatures at 1, 2 and 3 h after treatment in all five groups, we found no difference in mean temperatures between groups at any time point.
DISCUSSION
Hypothermia has been shown to non-specifically mitigate secondary HI injury cascades, but does not effectively inhibit these processes in all cases, indicating that a second therapy may be helpful in continuing hypothermia's beneficial effects and extending the neuroprotection. Ideally, this secondary treatment would be safe over an extended treatment period, covering the long period of secondary neuronal and possibly oligodendroglial loss after moderate and severe HI injury.
We have shown that the combination of NAC started with hypothermia after severe focal HI in PND 7 rats improves infarct and residual brain volumes at 48 h, 2 and 4 wk, and affords significantly more neuroprotection than either therapy alone. The infarct volume at 48 h was reduced to 55% of the untreated group with NAC treatment, and 44.6% with hypothermia treatment. These results are in accordance with earlier reports of hypothermia where the infarct area is reduced to about 50% compared with the vehicle (24,25). However, the combined treatment of systemic hypothermia and NAC significantly decreased total infarct volume to 8% of the untreated group, indicating that combination treatment significantly and synergistically attenuates the infarct size even in this severe HI injury model.
Previous studies have reported that neonatal HI injury retards the development of several neurological reflexes like ear twitch, grasping, gait, negative geotaxis and also results in delayed performance in righting, geotaxis and gait reflexes (22). We measured short-term functional neurological outcomes by cliff aversion and negative geotaxis, which tests labyrinthine and cerebellar integration (26). Both functional outcomes at day 7 were significantly better in the HYP + NAC group compared with vehicle treated, NAC or hypothermia treatment alone.
Longer-term outcomes may be suggested by evaluating myelination in neonatal brain as an important index of normal maturation and development (27). We concentrated on the corpus callosum and cingulum for preservation and recovery of white matter after HI injury since decrease in the size of the corpus callosum has been shown to adversely affect the motor co-ordination in the preterm neonates at a later age (28).
We examined markers of myelination up to 4 wk after the HI insult and found a persistent, marked improvement in the expression of positive myelin sheaths in the combination group (HYP + NAC), compared with the Vehicle, NAC or Hypothermia alone in the corpus callosum as well as cingulum areas of the cortex.
Although myelination has been clearly affected by HI injury and improved by combination treatment in our focal HI model, we did not see any differences in total numbers of cells in the two brain regions by Hoechst staining between vehicle and sham treated animals. In explanation of preservation of total cell numbers in these white matter regions, it may be that astroglial cells are increased in the vehicle treated HI animals, while other cell populations, such as oligodendrocytes are decreased, resulting in the total number of cells by Hoechst staining remaining unchanged. While we did not investigate the cell subpopulations responsible for this effect, earlier reports from our laboratory have shown that NAC prevents degeneration of oligodendrocyte progenitors and hypomyelination in developing rat brain in an endotoxin-induced periventricular leukomalacia (PVL) model (21). It may be inferred that oligodendrocyte progenitor cells are better preserved with combination treatment, but further studies will be needed to characterize cell sub-populations at different time points after recovery.
We noted continued brain volume loss up to 4 wk after severe HI injury, as have other investigators even with hypothermia treatment (14,29–31). Rats treated with NAC and hypothermia for 2 to 4 wk post-HI (Fig. 3C,D) were protected against this continued secondary cell death with brain volumes that were not different from sham animals during this tenuous period. Our data, therefore, suggest that certain mechanisms of brain injury, including oxidative injury, may be important to inhibit over longer periods. NAC may be especially effective as an adjunctive HI therapy in neonates due to their immature anti-oxidant systems, which are easily overwhelmed with the oxidative stress of HI injury.
In summary, we have shown important synergistic effects of short term hypothermia and longer term NAC treatment on multiple neurological outcome parameters in our immature rat model of focal HI brain injury. NAC has low toxicity, crosses the blood brain barrier (32), and is approved by the Food and Drug Administration for the treatment of acetaminophen toxicity and as a treatment for chronic bronchitis in adults and cystic fibrosis patients. Few therapies have shown similar long lasting neuropreservation with a safety profile which is favorable for prolonged administration.
Abbreviations
- HI:
-
hypoxia-ischemia
- LFB:
-
luxol fast blue
- MBP:
-
myelin basic protein
- NAC:
-
N-acetylcysteine
- PLP:
-
proteolipid protein
References
Levene MI, Sands C, Grindulis H, Moore JR 1986 Comparison of two methods of predicting outcome in perinatal asphyxia. Lancet 1: 67–69
Shankaran S, Woldt E, Koepke T, Bedard MP, Nandyal R 1991 Acute neonatal morbidity and long-term central nervous system sequelae of perinatal asphyxia in term infants. Early Hum Dev 25: 135–148
Simon NP 1999 Long-term neurodevelopmental outcome of asphyxiated newborns. Clin Perinatol 26: 767–778
Yager JY, Asselin J 1996 Effect of mild hypothermia on cerebral energy metabolism during the evolution of hypoxic-ischemic brain damage in the immature rat. Stroke 27: 919–925
Gunn AJ, Gunn TR, De Haan HH, Williams CE, Gluckman PD 1997 Dramatic neuronal rescue with prolonged selective head cooling after ischemia in fetal lambs. J Clin Invest 99: 248–256
Colbourne F, Corbett D 1994 Delayed and prolonged post-ischemic hypothermia is neuroprotective in the gerbil. Brain Res 654: 265–272
Laptook AR, Corbett RJ, Sterett R, Burns DK, Garcia D, Tollefsbol G 1997 Modest hypothermia provides partial neuroprotection when used for immediate resuscitation after brain ischemia. Pediatr Res 42: 17–23
Eicher DJ, Wagner CL, Katikaneni LP, Hulsey TC, Bass WT, Kaufman DA, Horgan MJ, Languani S, Bhatia JJ, Givelichian LM, Sankaran K, Yager JY 2005 Moderate hypothermia in neonatal encephalopathy: efficacy outcomes. Pediatr Neurol 32: 11–17
Eicher DJ, Wagner CL, Katikaneni LP, Hulsey TC, Bass WT, Kaufman DA, Horgan MJ, Languani S, Bhatia JJ, Givelichian LM, Sankaran K, Yager JY 2005 Moderate hypothermia in neonatal encephalopathy: safety outcomes. Pediatr Neurol 32: 18–24
Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, Polin RA, Robertson CM, Thoresen M, Whitelaw A, Gunn AJ 2005 Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet 365: 663–670
Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, Fanaroff AA, Poole WK, Wright LL, Higgins RD, Finer NN, Carlo WA, Duara S, Oh W, Cotten CM, Stevenson DK, Stoll BJ, Lemons JA, Guillet R, Jobe AH, National Institute of Child Health and Human Development Neonatal Research Network 2005 Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med 353: 1574–1584
Taylor DL, Mehmet H, Cady EB, Edwards AD 2002 Improved neuroprotection with hypothermia delayed by 6 hours following cerebral hypoxia-ischemia in the 14-day-old rat. Pediatr Res 51: 13–19
Colbourne F, Sutherland GR, Auer RN 1999 Electron microscopic evidence against apoptosis as the mechanism of neuronal death in global ischemia. J Neurosci 19: 4200–4210
Fairchild KD, Singh IS, Patel S, Drysdale BE, Viscardi RM, Hester L, Lazusky HM, Hasday JD 2004 Hypothermia prolongs activation of NF-{kappa}B and augments generation of inflammatory cytokines. Am J Physiol Cell Physiol 287: C422–C431
Adachi M, Sohma O, Tsuneishi S, Takada S, Nakamura H 2001 Combination effect of systemic hypothermia and caspase inhibitor administration against hypoxic-ischemic brain damage in neonatal rats. Pediatr Res 50: 590–595
Alkan T, Kahveci N, Buyukuysal L, Korfali E, Ozluk K 2001 Neuroprotective effects of MK 801 and hypothermia used alone and in combination in hypoxic-ischemic brain injury in neonatal rats. Arch Physiol Biochem 109: 135–144
Khan M, Sekhon B, Jatana M, Giri S, Gilg AG, Sekhon C, Singh I, Singh AK 2004 Administration of N-acetylcysteine after focal cerebral ischemia protects brain and reduces inflammation in a rat model of experimental stroke. J Neurosci Res 76: 519–527
Sekhon B, Sekhon C, Khan M, Patel SJ, Singh I, Singh AK 2003 N-Acetyl cysteine protects against injury in a rat model of focal cerebral ischemia. Brain Res 971: 1–8
Vannucci RC, Connor JR, Mauger DT, Palmer C, Smith MB, Towfighi J, Vannucci SJ 1999 Rat model of perinatal hypoxic-ischemic brain damage. J Neurosci Res 55: 158–163
Rice JE 3rd, Vannucci RC, Brierley JB 1981 The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol 9: 131–141
Paintlia MK, Paintlia AS, Barbosa E, Singh I, Singh AK 2004 N-acetylcysteine prevents endotoxin-induced degeneration of oligodendrocyte progenitors and hypomyelination in developing rat brain. J Neurosci Res 78: 347–361
Lubics A, Reglodi D, Tamas A, Kiss P, Szalai M, Szalontay L, Lengvari I 2005 Neurological reflexes and early motor behavior in rats subjected to neonatal hypoxic-ischemic injury. Behav Brain Res 157: 157–165
Reglodi D, Kiss P, Tamas A, Lengvari I 2003 The effects of PACAP and PACAP antagonist on the neurobehavioral development of newborn rats. Behav Brain Res 140: 131–139
Pabello NG, Tracy SJ, Keller RW 2004 Protective effects of brief intra- and delayed postischemic hypothermia in a transient focal ischemia model in the neonatal rat. Brain Res 995: 29–38
Zhu C, Wang X, Cheng X, Qiu L, Xu F, Simbruner G, Blomgren K 2004 Post-ischemic hypothermia-induced tissue protection and diminished apoptosis after neonatal cerebral hypoxia-ischemia. Brain Res 996: 67–75
Hermans RH, Hunter DE, Mcgivern RF, Cain CD, Longo LD 1992 Behavioral sequelae in young rats of acute intermittent antenatal hypoxia. Neurotoxicol Teratol 14: 119–129
Tanaka S, Mito T, Takashima S 1995 Progress of myelination in the human fetal spinal nerve roots, spinal cord and brainstem with myelin basic protein immunohistochemistry. Early Hum Dev 41: 49–59
Rademaker KJ, Lam JN, Van Haastert IC, Uiterwaal CS, Lieftink AF, Groenendaal F, Grobbee DE, de Vries LS 2004 Larger corpus callosum size with better motor performance in prematurely born children. Semin Perinatol 28: 279–287
Trescher WH, Ishiwa S, Johnston MV 1997 Brief post-hypoxic-ischemic hypothermia markedly delays neonatal brain injury. Brain Dev 19: 326–338
Li Y, Chopp M, Jiang N, Yao F, Zaloga C 1995 Temporal profile of in situ DNA fragmentation after transient middle cerebral artery occlusion in the rat. J Cereb Blood Flow Metab 15: 389–397
Grow JL, Liu YQ, Barks JD 2003 Can lateralizing sensorimotor deficits be identified after neonatal cerebral hypoxia-ischemia in rats?. Dev Neurosci 25: 394–402
Farr SA, Poon HF, Dogrukol-Ak D, Drake J, Banks WA, Eyerman E, Butterfield DA, Morley JE 2003 The antioxidants alpha-lipoic acid and N-acetylcysteine reverse memory impairment and brain oxidative stress in aged SAMP8 mice. J Neurochem 84: 1173–1183
Acknowledgements
The authors thank Ms. Joyce Bryan and Ms. Hope Terry for their help in arrangement of animals and chemicals. We also thank Ms. Carrie Barnes for providing histopathological support. We thank the Children's Research Institute for providing the laboratory space and the animal facility for carrying out the work.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by grants (NS-40144, NS-22576, NS-34741, NS-37766, and NS-40810) from the National Institutes of Health (NIH).
Rights and permissions
About this article
Cite this article
Jatana, M., Singh, I., Singh, A. et al. Combination of Systemic Hypothermia and N-acetylcysteine Attenuates Hypoxic-Ischemic Brain Injury in Neonatal Rats. Pediatr Res 59, 684–689 (2006). https://doi.org/10.1203/01.pdr.0000215045.91122.44
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/01.pdr.0000215045.91122.44