Abstract
The peptides platelet-derived growth factor-A (PDGF-A) and especially -B have important roles in lung development. The effect of hyperoxic exposure with and without inhaled nitric oxide (iNO) on lung expression of PDGF and its receptors is unknown. We hypothesized that hyperoxia exposure would suppress mRNA expression and protein production of these ligands and their receptors. The addition of iNO to hyperoxia may further aggravate the effects of hyperoxia. Thirteen-day-old piglets were randomized to breathe 1) room air (RA); 2) 0.96 fraction of inspired oxygen (O2), or 3) 0.96 fraction of inspired oxygen plus 50 ppm of NO (O2 + NO), for 5 d. Lungs were preserved for mRNA, Western immunoblot, and immunohistochemical analyses for PDGF-A and -B and their receptors PDGFR-α and -β. PDGF-B mRNA expression was greater than that of PDGF-A or PDGFR-α and -β in RA piglet lungs (p < 0.05). Hyperoxia with or without iNO reduced lung PDGF-B mRNA and protein expression relative to the RA group lungs (p < 0.01). PDGF-B immunostain intensity was significantly increased in the alveolar macrophages, which were present in greater numbers in the hyperoxia-exposed piglet lungs, with or without NO (p < 0.01). PDGFR-β immunostaining was significantly increased in airway epithelial cells in O2- and O2 + NO–exposed piglets. PDGF-A and PDGFR-α immunostain intensity and distribution pattern were unchanged relative to the RA group. Sublethal hyperoxia decreases PDGF-B mRNA and protein expression but not PDGF-A or their receptors in piglet lungs. iNO neither aggravates nor ameliorates this effect.
Similar content being viewed by others
Main
Platelet-derived growth factor (PDGF) is a mitogen for cells of mesenchymal origin, such as fibroblasts and smooth muscle cells. It exists as a family of homodimers—PDGF-AA, PDGF-BB, PDGF-CC, or PDGF-DD—and a heterodimer—PDGF-AB. These molecules bind to their cell surface receptors that also form either homodimers—PDGFR-αα and PDGFR-ββ—or a heterodimer—PDGFR-αβ (1–3). PDGF is important for both alveogenesis and angiogenesis of the normally developing lung (4). Expression of different forms of PDGF peaks in the canalicular stage, suggesting a possible role in the development of the gas-exchanging regions of the lung (5,6). In the canalicular and saccular stages, PDGF receptor expression is seen in epithelial and mesenchymal cells (7). Stimulation of PDGF receptors by PDGF ligands induces lung development (8). Although PDGF seems to be critical for normal lung development, the isoform PDGF-B expression is also associated with abnormal cellular hyperplasia and fibrosis in lungs that are exposed to hyperoxia (9–11) or to hypoxia (12). In both situations, PDGF-B has been shown to increase its expression, at both protein and mRNA levels, whereas PDGF-A and PDGF-B receptors react with inconsistent results (9–11). In mice deficient for PDGF-A expression, there is postnatal development of emphysema with failure of alveolar septation (5).
Hyperoxic injury to rapidly growing lungs contributes to development of chronic lung disease of prematurity. Hyperoxic damage to the lung is accompanied by inflammatory reactions such as leukocyte infiltration and cytokine release into the lung parenchyma (13). PDGF may be involved in this process because of its chemotactic and stimulatory effects to neutrophils and monocytes (14,15). As a mitogen, PDGF may also participate in the tissue-remodeling process after inflammation by stimulating fibroblast cell growth, resulting in collagen deposition and various degrees of lung fibrosis (16,17).
Infants with a variety of pulmonary disorders are now treated with the combination of fraction of inspired oxygen of ≥0.95 and inhaled nitric oxide (iNO) (18). Although the addition of iNO to supplemental oxygen has been shown to reduce the need for extracorporeal membrane oxygenation treatment, a significant percentage of survivors develop bronchopulmonary dysplasia, even in full-term infants (19). In addition, there are now published disparate results of recent clinical trials in which NO and O2 have been co-administered to preterm infants to prevent or ameliorate bronchopulmonary dysplasia. Schreiber et al. (20) demonstrated beneficial effects of iNO in preterm infants but only those who were less severely ill. In contrast, Van Mears et al. (21) and Macrae et al. (22) demonstrated no benefit of NO administration in preterm infants and indeed possible adverse events. It is likely that some preterm infants benefit from NO administration and some may have no benefit or demonstrate an exacerbation of pulmonary and other organ injury.
Whether iNO improves or aggravates oxygen toxicity has not been resolved. NO toxicity is usually attributed to its oxidative capabilities, especially that of its metabolites nitrogen dioxide (NO2) and peroxynitrite (23–25). In the presence of oxygen, NO reacts readily in a concentration-dependent manner to form peroxynitrite, a potent oxidant that is capable of oxidizing lipids and proteins. Peroxynitrite inactivates both α-1-antiprotease (26) and tissue inhibitors of metalloproteinases and may activate matrix metalloproteinases (27,28).
Garat et al. (29) demonstrated that survival of adult rats that were exposed to hyperoxia was not improved by iNO. However, inhalation of 10 ppm of NO, co-administered with O2, was associated with reduction of a marker of oxidative injury but had no effect on alveolar barrier permeability to protein. Inhalation of 100 ppm of NO increased vascular permeability to protein. Thus, iNO can either reduce or increase the early consequences of hyperoxic lung injury.
Nelin et al. (30) and Gutierrez et al. (31) also studied survival of adult rats with NO and O2 administration. Both demonstrated improvement in duration of survival with 100 ppm of NO; however, overall survival was very brief, with a median of only 51 h. Pulmonary alveolar cell culture studies (31) showed reduced injury with co-administration of NO and O2. Our group has shown that, at least compared with neonatal piglets (32), rat pups demonstrate substantial endogenous pulmonary NO production. Thus, the relevance of adult rat models to the questions raised in preterm infants is unclear.
Additional studies that have investigated the interaction of O2 and NO have evaluated the effects on surfactant biology. Issa et al. (33) demonstrated that iNO decreased hyperoxia-induced surfactant abnormality in rabbit pups that were delivered on day 19 of gestation and then were exposed to NO at 14 ppm for 20 h, beginning several hours after delivery. Compared with hyperoxia-exposed animals only, iNO prevented the effects of oxygen-induced reduction in the large surfactant aggregates and surface activity. In addition, surfactant protein B concentration in alveolar lavage fluid was relatively preserved in the NO studies. However, Robbins et al. (34) demonstrated that 48 h of exposure to 100% oxygen and to NO, in neonatal piglets, resulted in an exacerbation in the surfactant dysfunction as measured by minimum surface tension. From both these clinical and animal science-based results, it is clear that investigation in relevant animal models of clinically relevant doses of NO for clinically relevant periods of time continues to be important (35).
With this as background, we sought to analyze the effect of iNO on the expression of PDGF-A and -B mRNA and peptides and their receptors PDGFR-α and -β in lungs of animals that were exposed to sublethal oxygen toxicity, with or without concomitant iNO administration. We used a 13-d-old piglet model because, at this age, piglets have relatively mature lungs that demonstrate secondary septation and manifest rapid lung growth, achieving an average 25% increase in lung wet or dry weight in 1 wk (unpublished). This pattern—although not this rate—of growth is similar to that in term newborn human lungs (36). Because PDGF and its receptors have critical roles in lung morphogenesis and because hyperoxia contributes to the alveolar and microvascular hypoplasia found in bronchopulmonary dysplasia, we hypothesized that hyperoxic exposure would suppress expression of PDGF and of its receptors in rapidly growing lungs. We further sought to determine whether concurrent iNO administration would aggravate or ameliorate the hyperoxic injury.
METHODS
Animal exposure.
The animal exposure method has been reported previously (13,37). Briefly, we randomly separated 10- to 17-d-old piglets into three groups and placed them for 5 d in chambers that contained 1) room air (RA), 2) >95% oxygen (O2), or 3) O2 + 50 ppm of NO (O2 + NO). All animals weighed between 2.7 and 3.7 kg at the start of the exposure, and mean age (13.5 d) and mean weight (3.1 kg) were not different among the three groups.
Humidified medical grade O2 and NO (Puritan Bennett, Overland Park, KS) were introduced at flow rates sufficient to maintain chamber concentration at ≥96% and 50 ppm, respectively, throughout the experiment. Activated charcoal was used to scavenge CO2 and NO2. Gas concentration at chamber outlet port was monitored for O2, CO2, NO, and NO2. At the end of exposure, piglets were anesthetized with 2 mg/kg xylazine, 20 mg/kg ketamine, and 40 mg/kg sodium pentobarbital and killed with a high dose of pentobarbital. Lungs were harvested promptly. The lower and middle lobes of the right lung were lavaged with six aliquots of 25 mL of PBS. Part of the left lung was cut into small pieces, quickly frozen in liquid nitrogen, and stored at −80°C for protein and RNA studies. The upper lobe of the right lung was fixed in 4% formalin and used for immunohistochemical analysis.
Immunohistochemistry.
Formalin-fixed, paraffin-embedded lung tissues were cut into 4-μm sections and mounted on positively charged glass slides. After deparaffinization and rehydration, sections were incubated with rabbit polyclonal antibodies against PDGF peptides and PDGFR-α or a mouse MAb against PDGFR-β (Santa Cruz Biotechnology, Santa Cruz, CA), followed by rabbit or mouse biotinylated secondary antibodies and then a preformed avidin-biotinylated horseradish peroxidase macromolecular complex (Vectastain ABC Elite Kit; Vector Laboratories, Burlingame, CA). Antigens were visualized with 3, 3′-diaminobenzidine substrate (Sigma Chemical Co., St. Louis, MO). The immunostains were semiquantified with light microscopy by two observers who were blinded to treatment group, using a 0–3 scoring system, as follows: 0 = no staining; 1 = light staining; 2 = moderate staining; and 3 = heavy staining. Observations of the two observers were averaged.
Western blot analysis.
Piglet lung tissue was homogenized in RIPA buffer that contained 1× PBS; 0.1% SDS; 1% Igepal CA-630 (Sigma Chemical Co.); 0.5% sodium deoxycholate; and protease inhibitors, including 10 μg/g tissue PMSF, 30 μL/mL Aprotinin (Sigma Chemical Co.; cat. no. A6279), and 10 μL/mL 100 mM of sodium orthovanadate. The homogenate was centrifuged for 10 min at 10,000 × g (4°C). The supernatant was applied to a 10% Bis-Tris acrylamide gel with 40 μg protein/lane. After electrophoresis, proteins were transferred to a nitrocellulose membrane and the membrane was carried on for Western blot. Reagents used in the procedure were a nonspecific blocking buffer Western Breeze (Invitrogen kit, cat. no. 46-7003, 46-7004); rabbit antihuman polyclonal PDGF-A, PDGFR-α, and PDGF-B and mouse antihuman MAb to PDGFR-β were obtained from (Santa Cruz Biotechnology; cat. no. SC-128, SC-338, SC-127, and SC-6252, respectively). β-Actin antibody was purchased from (Santa Cruz Biotechnology) as was secondary antibody conjugated to horseradish peroxidase. Detection was by an enhanced chemiluminescent technique (Amersham Biosciences, Piscataway, NJ; cat no. RPN2106). Western blot results were analyzed quantitatively using a densitometer and the Image-Quant software (Amersham Biosciences). Values were expressed as a ratio of signal of interest to internal standard, β-actin.
Cloning of pig mRNA sequences.
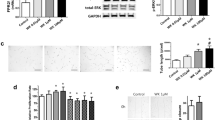
Degenerate oligonucleotide primers (20 bp) for PDGF-A and -B, PDGF receptors (PDGFR-α and -β), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were synthesized on the basis of the published sequences (PubMed) for human, rat, and mouse. Piglet (Sus scrofa) lung tissue total RNA was isolated using Tri Reagent (Sigma Chemical Co.) and subjected to reverse transcription (RT) using RETROscript reverse transcription kit (Ambion Diagnostics, Austin, TX). PCR was carried out using the degenerate primers and the reverse transcribed cDNA. PCR products of the appropriate size were cloned into pCR II-TOPO vector (Invitrogen Corp., Carlsbad, CA) and sequenced. Sequence results were analyzed with BLAST on PubMed. BLAST results and sequence comparisons of PDGF molecules are compared (Fig. 1), which indicates high sequence homology (ranging from 76% to 92%) between cloned pig sequences and sequences of other species.
Quantitative analysis of lung tissue mRNA using real-time PCR technique.
On the basis of cloned sequences from piglet, PCR primers were synthesized for PDGF-A, PDGF-B, PDGFR-α, PDGFR-β, and pig GAPDH with designated PCR products for 200 bp. Isolated pig lung tissue total RNA was treated with DNA Free Kit (Ambion Diagnostics) to remove any residual DNA contamination and was reverse-transcribed using the RETROscript kit. The RT product was diluted 5-, 50-, 500-, and 5000-fold, and each was subjected to quantitative PCR analysis using the AmpliTag Gold DNA polymerase and SYBR Green I (Applied Biosystems, formerly PE Biosystems, Foster City, CA) on iCycler (Bio-Rad Laboratories, Hercules, CA). Primers that yielded a linear relationship between RT dilutions and PCR quantification were selected for real-time quantification experiment. Appropriate template dilutions (1:10–1:100 for most experiments) were decided at the same time. The GAPDH was used as a housekeeping gene for the analysis of PDGFs or PDGF receptors, the genes of interest. GAPDH was used on the basis of observations of others that its expression is not significantly affected by exposure to oxidizing agents (38) and because of our own findings of no significant difference among groups in GAPDH mRNA threshold cycle of exponential increase. Each experimental group for real-time quantitative analysis on iCycler included both the housekeeping gene and the genes of interest. The relative quantity of mRNA for each experimental group was calculated with the following equation: Relative mRNA = 2 − (threshold cycle of genes of interest − threshold cycle of housekeeping gene).
Statistics.
Statistical analysis was performed using either MS-Excel or INSTAT (GraphPad Software, San Diego, CA). Parametric data were analyzed with one-way ANOVA, and nonparametric data were analyzed with Kruskal-Wallis test. A p < 0.05 was considered significant. Results are expressed as mean ± SD unless noted otherwise.
RESULTS
mRNA analysis.
Quantitative mRNA analysis by real-time PCR technique showed that in RA breathing piglets, lung PDGF-B mRNA expression is 5- to 10-fold higher than that of PDGF-A and of the receptors PDGFR-α and -β (p < 0.01; Fig. 2). Breathing hyperoxia with or without NO resulted in a significant decline in lung PDGF-B mRNA expression compared with RA control (p < 0.001). There was no significant difference in the suppressive effect of hyperoxia alone versus that of hyperoxia with concurrent iNO on lung PDGF mRNA expression. Hyperoxia with or without NO breathing did not significantly alter mRNA expression of PDGF-A or of the receptors PDGFR-α and -β. There was no significant difference in initiation of exponential doubling cycle for GAPDH mRNA among the three groups when the same initial amount of total RNA was present.
Quantitative mRNA analysis using real-time PCR. There is a significant difference between PDGF-B and PDGF-A and the α and β receptors. The mRNA level of PDGF-B is 5- to 10-fold higher than PDGF-A and PDGFR-α and -β proteins in RA. The presence of O2, with or without NO, significantly reduced PDGF-B mRNA expression compared with RA controls (p < 0.001). NO did not seem to modify the effect of hyperoxia. n = 5, 4, and 4, respectively, for the three groups.
PDGF-A, PDGF-B, and Western blot analysis.
Results of Western blot analyses of lung PDGF-A and PDGF-B proteins paralleled the changes seen in mRNA expression (Figs. 3 and 4). PDGF-B protein expression declined significantly in the lungs of both the piglets with hyperoxia and the piglets with hyperoxia and NO breathing compared with RA controls (p < 0.05). PDGF-A protein expression was unchanged.
Immunohistochemical analysis.
Intense immunostaining reactivity of airway epithelial cells, from the bronchi to the alveolar ducts, as well as of septal wall cells, vascular endothelium, and alveolar macrophages, was seen for both PDGF peptides and their receptor proteins PDGFR-α and -β in all RA-exposed piglet lungs (Fig. 5). For PDGF-A and the α receptor, in the presence of RA, O2, and O2 + NO, the immunostaining intensity score was high, and the distribution did not change in the epithelium, endothelium, macrophage, or alveolar septa. These findings indicate that the PDGF ligand and receptors are constitutively near maximally up-regulated at this stage of lung growth and O2 or O2 + NO did not influence this result. Typical staining at the bronchiolar level is shown in Fig. 6.
PDGF-A and PDGF-B and PDGFR-α did not significantly change in airway epithelium or vascular endothelium in O2- or O2 + NO– compared with RA-treated animals. However, PDGFR-β reactivity was significantly increased (p < 0.05) in the macrophages that were exposed to O2 and O2 + NO. The expression of PDGFR-β was significantly increased in the epithelium at all levels of the airway and in the alveolar septa in the O2- and O2 + NO–exposed animals; n = 5, 4, and 4 for RA, O2, and O2 + NO, respectively.
Immunohistochemical staining of piglet lung. (A) O2-treated sample. Bronchiole epithelium (indicated by arrow) is stained intensely (score 3) with anti–PDGF-B antibody. An alveolar macrophage (arrowhead) is also intensely stained. (B) RA group sample showing bronchiole epithelium. No anti–PDGF-B staining is observed (score 0). Scale bar = 6 μm.
PDGF-B immunoreactivity expression did not significantly change with exposure to hyperoxia or with hyperoxia + NO, except in macrophages. Oxygen and O2 + NO significantly increased both the number of macrophages and the intensity of the expression (p < 0.01 and < 0.05, respectively). It is interesting that the expression of PDGFR-β significantly increased in the epithelium at all levels of the airways in the O2- and O2 + NO–exposed animals (p < 0.05; Fig. 5). It also increased significantly in the alveolar septum (p < 0.05). There were also increases in the vascular endothelium and macrophage staining intensity, but they did not reach significance.
DISCUSSION
Various studies have focused on adult animal models to correlate O2-mediated pulmonary cellular hyperplasia to changes in expression of various growth factor genes. Such models are simplistic in that the adult lung being a nonproliferating organ is unlikely to exhibit dramatic alterations in growth factor expression after the onset of a proliferative lung insult. In contrast, the neonatal lung is an actively proliferating organ, even in the absence of injury. However, very few studies have focused on the injury-mediated changes in the neonatal lung.
In this study, we investigated the effect of hyperoxia with or without concurrent iNO on expression of lung PDGF peptides and their receptors in the rapidly growing neonatal lung. We found that hyperoxia with concurrent iNO, like hyperoxic breathing alone for 5 d, had a suppressive effect on lung PDGF-B mRNA transcripts and protein expression but had no effect on PDGF-A or on both PDGF receptors expression, as assessed in total lung homogenates. However, we also found increased immunostaining intensity of PDGF-B in macrophages and of the receptor PDGFR-β in alveolar septa and airway epithelial cells. These latter results suggest that there was up-regulated expression in response to hyperoxia with or without iNO.
PDGF peptides and their receptors have been studied in the lungs of adult and immature animals that were treated with hyperoxia but not in the lungs of young growing animals (10). Full-term human infants who experience pulmonary hypertension are treated with hyperoxia and iNO for several days' duration (18,19). However, the effect of administering fraction of inspired oxygen of 0.95 with concurrent iNO on expression of lung PDGF peptides and their receptors has not been examined in animals of any age. The results from the current study provide the first insight into the potential implications of such use on PDGF-regulated events in the growing lung. Previous investigators have shown that the adult rat has low constitutive lung levels of PDGF-B mRNA and that, in the presence of hyperoxia, PDGF-B mRNA is elevated 2.5-fold by day 3 and remains high for 7 d. This early rise in mRNA precedes the increase in protein synthesis and other responses that reflect remodeling with hyperoxia, suggesting that PDGF proteins modulate lung injury and repair (9). Powell et al. (10), studying hyperoxia in adult rats, showed that within hours of exposure, mRNA levels of the α and β receptors increase, return to normal by 1 d, and decline thereafter in the presence of hyperoxia. The PDGF-A mRNA also rose within hours and then returned to normal levels, even though the hyperoxic exposure continued. In their study, the PDGF-B chain mRNA also responded to hyperoxia by increasing 10-fold on day 3 of exposure. Again, this increase preceded the day 4 proliferative response of microvascular adventitial fibroblasts, precursor smooth muscle cells, and epithelial cells. In adult rats with acute lung injury from bleomycin, the mitogenic potency of PDGF-B was more than 5 times that of PDGF-A (26). Furthermore, the role of PDGF-BB as a critical growth factor in early postnatal rat lung growth has been demonstrated by Buch et al. (39) who, using both neutralizing antibodies to PDGF-BB and a truncated soluble PDGFR-β, observed a significant reduction of DNA synthesis in the first week of life. They showed that newborn rats that breathed RA had a significant rise in lung PDGF-B, PDGF-A, and PDGFR-β mRNA at 6 d of life but a decrease in PDGFR-α mRNA, compared with levels at birth (day 0). Negligible PDGF-B immunoreactivity was seen at birth, increasing by day 4, declining at day 6, and increasing again at day 10 of life. Similarly, PDGF-A and the α receptor immunoreactivity varied from 0 to 14 d, with the intensity and distribution changing as various tissue elements, e.g. epithelium and microvasculature matured and underwent growth and differentiation (39). Exposure of these newborn rats to 60% O2 significantly increased the mRNA for PDGF-B and that of the α and β receptor but not PDGF-A. PDGF-A and -B and the α receptor immunoreactivity were reduced and delayed in hyperoxia, consistent with overall inhibition of lung growth (39). Evidence of a role for PDGF-B in lung growth was shown by a reduction in lung DNA synthesis after treatment with PDGF-BB neutralizing antibodies.
The expression of PDGF and its receptors in the immature lung in the presence of hyperoxia is much more complicated than in the mature adult lung because of the rapid growth and tissue remodeling and differentiation that occurs with immaturity. Therefore, it can be anticipated that experiments on immature rats, lambs, piglets, and baboons may give various PDGF responses to hyperoxia, depending on the stage of development and the species.
In our study, after 5 d of hyperoxia, with or without NO, the piglet showed a significant decrease in mRNA expression of PDGF-B mRNA but not PDGF-A mRNA, or PDGFR-α, or PDGFR-β. This was paralleled by a significant fall in the PDGF-B protein in whole-lung homogenate. The PDGF-A protein did not change. These findings suggest that this relatively short but potent hyperoxic stimulus depressed the mitogenic effect of PDGF-B. However, within 48 h of stopping the hyperoxia, the PDGF-B protein, as determined by Western blot analysis, had recovered to control levels (data not shown). NO did not seem to have an independent significant influence on the levels of PDGF messenger or protein.
Our piglet model is in a more rapid lung growth phase than the adult animal but is unlike the neonatal rat model of Buch et al. (39). The piglet is analogous to the human full-term infant, which is also in a rapid lung growth phase, but the piglet lung is more mature.
The increased immunostaining intensity that we observed for PDGF-B and the β receptor in certain lung tissue and cell types after exposure to hyperoxia with or without NO at first would seem to conflict with our findings from hybridization studies of the lung homogenates. However, this seeming discrepancy between results from hybridization studies of the total lung homogenate and immunohistochemical analysis should not be surprising. Changes in lung tissue and cell-specific level of expression of a protein can occur in response to a stimulus and may not be consistent with results determined from studying the homogenate (40). This could arise if there are changes in cell population or in their numbers. The lung is made of many cell types (41). The capillary endothelial cells constitute two thirds of the parenchymal cell population, and the alveolar epithelial cells represent less than one third. Hyperoxia is known to alter cell types and numbers in the lungs and especially has a propensity to damage vascular endothelial and airway epithelial cells. Indeed, we found a significant rise in the number of alveolar macrophages and their immunostaining intensity for PDGF-B, in the hyperoxia-exposed piglet lungs, with or without NO, compared with the RA group.
Limitations of the current study include the limited number of time points evaluated and dose of NO studied. However, these notwithstanding, the study identifies perturbations that occur in lung expression of PDGF-B and its receptors in response to combined NO-hyperoxia exposure and therefore offers insight into potential complications of such use on lung growth and development.
In summary, hyperoxia with or without iNO suppresses total lung PDGF mRNA and protein expression in young growing piglet lungs. Concurrent iNO at 50 ppm does not aggravate or ameliorate these effects of hyperoxia on PDGF peptides or on expression of their receptors in this sublethal oxygen exposure model. Alterations in levels of lung PDGF could have permanent consequences for lung growth and development after the exposure period is completed. Confirming this possibility will require longer term follow-up studies.
Abbreviations
- GAPDH:
-
glyceraldehyde-3-phosphate dehydrogenase
- iNO:
-
inhaled nitric oxide
- NO:
-
nitric oxide
- PDGF:
-
platelet-derived growth factor
References
Heldin CH, Westermark B 1999 Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev 79: 1283–1316
Ostman A, Heldin CH 2001 Involvement of platelet-derived growth factor in disease: development of specific antagonists. Adv Cancer Res 80: 1–38
Betsholtz C, Karlsson L, Lindahl P 2001 Developmental roles of platelet-derived growth factors. Bioessays 23: 494–507
Lindahl P, Bostrom H, Karlsson L, Hellstrom M, Kalen M, Betsholtz C 1999 Role of platelet-derived growth factors in angiogenesis and alveogenesis. Curr Top Pathol 93: 27–33
Bostrom H, Willetts K, Pekny M, Leveen P, Lindahl P, Hedstrand H, Pekna M, Hellstrom M, Gebre-Medhin S, Schalling M, Nilsson M, Kurland S, Tornell J, Heath JK, Betsholtz C 1996 PDGF-A signaling is a critical event in lung alveolar myofibroblast development and alveogenesis. Cell 85: 863–873
Buch S, Jones C, Sweezey N, Tanswell K, Post M 1991 Platelet-derived growth factor and growth-related genes in rat lung. I. Developmental expression. Am J Respir Cell Mol Biol 5: 371–376
Han RN, Liu J, Tanswell AK, Post M 1993 Ontogeny of platelet-derived growth factor receptor in fetal rat lung. Microsc Res Tech 26: 381–388
Souza P, Tanswell AK, Post M 1996 Different roles for PDGF-alpha and -beta receptors in embryonic lung development. Am J Respir Cell Mol Biol 15: 551–562
Fabisiak JP, Evans JN, Kelley J 1989 Increased expression of PDGF-B (c-sis) mRNA in rat lung precedes DNA synthesis and tissue repair during chronic hyperoxia. Am J Respir Cell Mol Biol 1: 181–189
Powell PP, Wang CC, Jones R 1992 Differential regulation of the genes encoding platelet-derived growth factor receptor and its ligand in rat lung during microvascular and alveolar wall remodeling in hyperoxia. Am J Respir Cell Mol Biol 7: 278–285
Han RN, Buch S, Freeman BA, Post M, Tanswell AK 1992 Platelet-derived growth factor and growth-related genes in rat lung. II. Effect of exposure to 85% O2 . Am J Physiol 262: L140–L146
Berg JT, Breen EC, Fu Z, Mathieu-Costello O, West JB 1998 Alveolar hypoxia increases gene expression of extracellular matrix proteins and platelet-derived growth factor-B in lung parenchyma. Am J Respir Crit Care Med 158: 1920–1928
Ekekezie II, Thibeault DW, Zwick DL, Rezaiekhaligh MH, Mabry SM, Morgan RE, Norberg M, Truog WE 2000 Independent and combined effects of prolonged inhaled nitric oxide and oxygen on lung inflammation in newborn piglets. Biol Neonate 77: 37–44
Deuel TF, Senior RM, Huang JS, Griffin GL 1982 Chemotaxis of monocytes and neutrophils to platelet-derived growth factor. J Clin Invest 69: 1046–1049
Tzeng DY, Deuel TF, Huang JS, Baehner RL 1985 Platelet-derived growth factor promotes human peripheral monocyte activation. Blood 66: 179–183
Wilson E, Laster SM, Gooding LR, Lambeth JD 1987 Platelet-derived growth factor stimulates phagocytosis and blocks agonist-induced activation of the neutrophil oxidate burst: a possible cellular mechanism to protect against oxygen radical damage. Proc Natl Acad Sci USA 84: 2213–2217
Moore AM, Buch S, Han RN, Freeman BA, Post M, Tanswell AK 1995 Altered expression of type I collagen, TGF-beta 1, and related genes in rat lung exposed to 85% O2 . Am J Physiol 268: L78–L84
Kinsella JP, Truog WE, Walsh WF, Goldberg RN, Bancalari E, Mayock DE, Redding GJ, de Lemos RA, Sardesai S, McCurnin DC, Moreland SG, Cutter GR, Abman SH 1997 Randomized, multicenter trial of inhaled nitric oxide and high-frequency oscillatory ventilation in severe, persistent pulmonary hypertension of the newborn. J Pediatr 131: 55–62
Truog WE 1998 Inhaled nitric oxide: a tenth anniversary observation. Pediatrics 101: 696–697
Schreiber MD, Gin-Mestan K, Marks JD, Huo D, Lee G, Srisuparp P 2003 Inhaled nitric oxide in premature infants with the respiratory distress syndrome. N Engl J Med 349: 2099–2107
Van Meurs KP, Stevenson DK, Ehrenkranz RA, Lemons JA, Poole WK, Perritt R, Higgins RD, Wright LL, Neonatal Research Network 2004 Inhaled nitric oxide for preterm infants with severe respiratory failure. Pediatr Res 55: LB12, 4A
Macrae DJ, Field D, Mercier JC, Moller J, Stiris T, Biban P, Cornick P, Goldman A, Gothberg S, Gustafsson LE, Hammer J, Lonnqvist PA, Sanchez-Luna M, Sedin G, Subhedar N 2004 Inhaled nitric oxide therapy in neonates and children: reaching European consensus. Intensive Care Med 30: 372–380
Gaston B, Drazen JM, Loscalzo J, Stamler JS 1994 The biology of nitrogen oxides in the airways. Am J Respir Crit Care Med 149: 538–551
Moncada S, Palmer RM, Higgs EA 1991 Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev 43: 109–142
Stamler JS, Singel DJ, Loscalzo J 1992 Biochemistry of nitric oxide and its redox-activated forms. Science 258: 1898–1902
Moreno JJ, Pryor WA 1992 Inactivation of α 1-proteinase inhibitor by peroxynitrite. Chem Res Toxicol 5: 425–431
Stricklin GP, Hoidal JR 1992 Oxidant-mediated inactivation of TIMP. Matrix Suppl 1: 325
Rajagopalan S, Meng XP, Ramasamy S, Harrison DG, Galis ZS 1996 Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro. Implications for atherosclerotic plaque stability. J Clin Invest 98: 2572–2579
Garat C, Jayr C, Eddahibi S, Laffon M, Meignan M, Adnot S 1997 Effects of inhaled nitric oxide or inhibition of endogenous nitric oxide formation on hyperoxic lung injury. Am J Respir Crit Care Med 155: 1957–1964
Nelin LD, Welty SE, Morrisey JF, Gotuaco C, Dawson CA 1998 Nitric oxide increases the survival of rats with a high oxygen exposure. Pediatr Res 43: 727–732
Gutierrez HH, Nieves B, Chumley P, Rivera A, Freeman BA 1996 Nitric oxide regulation of superoxide-dependent lung injury: oxidant-protective actions of endogenously produced and exogenously administered nitric oxide. Free Radic Biol Med 21: 43–52
Kaftan HA, Clark PL, Norberg M, Garg U, Thibeault DW, Truog WE 2003 Endogenous production of nitric oxide in endotoxemic piglets. Biol Neonate 83: 42–48
Issa A, Lappalainen U, Kleinman M, Bry K, Hallman M 1999 Inhaled nitric oxide decreases hyperoxia-induced surfactant abnormality in preterm rabbits. Pediatr Res 45: 247–254
Robbins CG, Davis JM, Merritt TA, Amirkhanian JD, Sahgal N, Morin FC 3rd, Horowitz S 1995 Combined effects of nitric oxide and hyperoxia on surfactant function and pulmonary inflammation. Am J Physiol 269: L545–L550
Truog WE, Castor CA, Sheffield MJ 2003 Neonatal nitric oxide use: predictors of response and financial implications. J Perinatol 23: 128–132
Winkler GC, Cheville NF 1985 Morphometry of postnatal development in the porcine lung. Anat Rec 211: 427–433
Youssef JA, Thibeault DW, Rezaiekhaligh MH, Mabry SM, Norberg MI, Truog WE 1999 Influence of inhaled nitric oxide and hyperoxia on Na,K-ATPase expression and lung edema in newborn piglets. Biol Neonate 75: 199–209
Cho M, Hung TK, Hussain MZ 2001 Hydrogen peroxide stimulates macrophage vascular endothelial growth factor release. Am J Physiol 280: H2357–H2363
Buch S, Han RN, Cabacungan J, Wang J, Yuan S, Belcastro R, Deimling J, Jankov R, Luo X, Lye SJ, Post M, Tanswell AK 2000 Changes in expression of platelet-derived growth factor and its receptors in the lungs of newborn rats exposed to air or 60% O2 . Pediatr Res 48: 423–433
Walsh J, Absher M, Kelley J 1993 Variable expression of platelet-derived growth factor family proteins in acute lung injury. Am J Respir Cell Mol Biol 9: 637–644
Crapo JD, Barry BE, Gehr P, Bachofen M, Weibel ER 1982 Cell number and cell characteristics of the normal human lung. Am Rev Respir Dis 126: 332–337
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported, in part, by NIH R-01 HL58125 (W.E.T.) and by K23 HL 04264 (I.I.E.).X.Z. is currently affiliated with the Department of Anatomy and Cell Biology, University of Kansas Medical Center, Kansas City, KS 66160.
Rights and permissions
About this article
Cite this article
Zhang, X., Reinsvold, P., Thibeault, D. et al. Responses of Pulmonary Platelet-Derived Growth Factor Peptides and Receptors to Hyperoxia and Nitric Oxide in Piglet Lungs. Pediatr Res 57, 523–529 (2005). https://doi.org/10.1203/01.PDR.0000155762.91748.8D
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/01.PDR.0000155762.91748.8D