Abstract
Chorioamnionitis and funisitis are associated with neonatal morbidity and mortality. We hypothesized that chorioamnionitis may stress fetal endothelium, activate proinflammatory gene transcription. and affect angiogenic homeostasis in fetal capillaries. Placentas from preterm infants were stained for heat-shock protein 70, nuclear factor-κB, hypoxia-inducible factor-1α, and vascular endothelial growth factor (VEGF). VEGF receptors (VEGF-R) 1 and 2 as well as the receptor tyrosine kinase with immunoglobulin and epidermal growth factor homology domains (TIE-2), which is involved in vascular remodeling, were quantified. Immunohistochemistry was analyzed by counting positive capillaries in placental terminal villi. Staining intensity was quantified by a three-step semiquantitative scale. The samples were divided into three matched groups according to histology: chorioamnionitis with funisitis (“funisitis”), chorioamnionitis without funisitis (“chorioamnionitis”), and control group with no inflammation. In tissues from the funisitis or chorioamnionitis group, heat-shock protein 70 expression was increased over the control group. More nuclear factor-κB–positive nuclei of endothelial cells in capillaries were counted in the funisitis and chorioamnionitis groups. Expression of VEGF and VEGF-R1 and -R2 were reduced in cases of funisitis or chorioamnionitis in comparison with controls. Hypoxia-inducible factor-1α expression tended to be slightly lower in the funisitis and chorioamnionitis groups but did not reach statistical significance. We speculate that cellular stress and changes in angiogenic homeostasis induced by proinflammatory activation of fetal endothelium in chorioamnionitis may not be limited to the placenta but may also involve other fetal organs.
Similar content being viewed by others
Main
Chorioamnionitis is very common in preterm birth (1). Microbial infection of the amnion and chorion are detected in 60% of all preterm deliveries (2). Funisitis is diagnosed as an infiltration of neutrophils in the umbilical cord vessels (3) and can be considered as a progression of chorioamnionitis with systemic inflammatory response syndrome of the fetus (4).
Although fetal inflammation reduces the incidence of respiratory distress syndrome (5,6) and increases the survival of preterm infants (7), the fetal inflammatory response has been associated with increased neonatal morbidity. The mechanisms by which fetal inflammation increases the incidence of bronchopulmonary dysplasia (BPD) (8), retinopathy of prematurity (9), intracranial hemorrhage (10), cerebral palsy, and adverse neurodevelopmental outcome (11–13) are not fully understood. Recently, a new potential mechanism in the pathogenesis of increased morbidity was put forward. The importance of impaired angiogenesis in the development of organs and BPD was shown by a reduced expression of proangiogenic factors and their receptors in lung tissue from preterm infants with BPD (14,15).
De novo synthesis of blood vessels (vasculogenesis) accounts for the major part of new blood vessel formation in the placenta during the first trimester, which is followed by angiogenesis (growth of existing vessels) in the next trimesters (16). Angiogenesis is a complex process in which the balance of agonists and antagonists drive growth, differentiation, survival, and remodeling of endothelial cells and their precursors (17). During the complete gestational period, fetal capillaries grow in the placenta toward the decidua (18). It is unclear whether a bacterial infection of the placenta—like in chorioamnionitis–affects the angiogenesis during gestation. In this study, we focused on the inflammatory changes on endothelial cell homeostasis and on selected angiogenic factors expressed by capillaries of the placenta of preterm infants that were exposed to chorioamnionitis or funisitis. We used chorioamnionitis and funisitis as criteria to characterize the prenatal inflammation to which the fetus was exposed. These histologic diagnoses are readily available in clinical practice and are important for outcome (19). We hypothesized that inflammation in chorioamnionitis and/or funisitis may impair cellular homeostasis in endothelial cells that may activate heat-shock protein 70 (HSP70). HSP70 is an unspecific marker of cellular stress that can be induced by hypoxic, oxidative, or osmotic stress (20). In addition, we evaluated the expression of hypoxia-inducible factor-1α (HIF-1α), which can be induced by hypoxia or ischemia (21,22). HIF-1α and HSP70 are expressed in placental endothelial cells throughout pregnancy (23) and interfere with proinflammatory gene transcription (24). Proinflammatory gene transcription can be induced by activation of nuclear factor-κB (NF-κB), which is a fundamental feature in inflammatory events in monocytes and endothelial cells (25–27). We evaluated the expression of NF-κB in placental endothelial cells that can induce proinflammatory gene transcription for interleukin (IL)-1 and tumor necrosis factor-α, which are known to be up-regulated in chorioamnionitis (4). The effects of inflammation on angiogenesis were evaluated by staining the placenta tissue for the expression of vascular endothelial growth factor (VEGF), which induces endothelial cell proliferation, sprouting, and tubulogenesis (28). VEGF can be induced by HIF-1 α or many oncogenes (21,22,29) and is closely related to the regulation of NF-κB by inhibiting IκB kinase activity (26). The effects of VEGF are mediated in part by the VEGF receptors (VEGF-R) 1 and 2 (30), which were detected in the placenta throughout gestation (31). In addition, the receptor tyrosine kinase with immunoglobulin and epidermal growth factor homology domains-2 (TIE-2) was quantified in the placenta. TIE-2 stimulates angiogenesis and vascular remodeling by endothelial cell migration and stabilizes newly formed vascular networks by recruiting periendothelial support cells and formation of extracellular matrix (32).
METHODS
Patients and study design.
Umbilical cord blood, umbilical cord, and placenta samples from 32 preterm infants [13 female, 19 male; gestational age median 26 wk (95% confidence interval [CI] 23–31 wk); birth weight median 925 g (95% CI 568–1752 g)] of a collection of 57 samples that were obtained from the Children's Hospital of the City of Cologne in 1998–2001 were analyzed (33). In the study of D'Alquen et al. (33), umbilical cord sections and umbilical blood samples of the same patients were analyzed for the expression of adhesion molecules. In this study, only funisitis resulted in increased expression and shedding of endothelial adhesion molecules, which correlated with increased concentrations of proinflammatory cytokines in umbilical blood samples (33).
Prenatal steroid prophylaxis was defined as an inclusion criterion. The patients were grouped according to histologic diagnosis of placenta and umbilical cord. Hematoxylin-eosin–stained sections of placenta and umbilical cord were reviewed by a pathologist who was unaware of clinical data. Chorioamnionitis was defined as infiltration of neutrophils in the extraplacental membranes or chorionic plate (34). Funisitis was diagnosed when neutrophils infiltrated the wall of umbilical cord vessels or Wharton's jelly (35). The first group of patients with chorioamnionitis and funisitis was referred to as funisitis group (n = 11). The second group had a diagnosis of chorioamnionitis without inflammation of the umbilical cord and therefore was referred to as chorioamnionitis group (n = 9). The control group showed no inflammation of placenta and umbilical cord (n = 12).
Characteristics of patients are shown in Table 1. There was no difference in distribution of gender, gestational age, birth weight, Apgar score after 5 min, and umbilical cord artery pH between groups. Almost all mothers in all three groups had uncontrollable preterm labor with no difference between groups. The funisitis group had the highest incidence of premature rupture of membranes, and the control group had the lowest (p < 0.05). The incidence of premature rupture of membranes was not different between the funisitis and chorioamnionitis groups. Pregnancies with pathologic changes in the placenta, such as gestational diabetes or preeclampsia, were excluded. The study was approved by the hospital ethics committee, and patients' parents gave informed consent.
Immunohistochemistry.
Immunohistochemical staining was performed following standard procedures. In brief, sections were cut from formalin-fixed, paraffin-embedded placenta samples at 2 μm and mounted on 3-amino-propyltriethoxy-silane–coated slides (Roth, Karlsruhe, Germany). After dewaxing and rehydration in graded ethanol solutions, antigen retrieval was carried out with 10 min of boiling in citric acid buffer (pH 6.0), using a microwave (750 W). Endogenous peroxidase activity was inhibited by 3% hydrogen peroxide in methyl alcohol. Nonspecific binding was blocked by incubation in PBS with 1% goat-serum and 1% BSA. Slides were incubated overnight at 4°C in a humidified chamber with primary antibodies at appropriate dilution in PBS. Primary antibodies were anti-human NF-κB (diluted 1:2000; sc-226; Santa Cruz Biotechnology, Santa Cruz, CA) and anti-human HSP70 (diluted 1:75; clone 289M; Biogenex, San Ramon, CA), anti-human VEGF (diluted 1:50; clone sc-152; Santa Cruz Biotechnology), anti-human VEGF-R1 (diluted 1:500; clone RP 077; Zytomed, Berlin, Germany), anti-human VEGF-R2 (diluted 1:500; clone AF 357; R&D Systems, Minneapolis, MN), anti-human TIE-2 (diluted 1:500; clone AF 313; R&D Systems), and anti-human HIF-1α (diluted 1:100; clone H1α67; Novus Biologicals, Littleton, CO). Detection was done with a goat anti-mouse secondary antibody conjugated to horseradish peroxidase (HRP; Dako, Glostrup, Denmark). The enzymatic reaction was developed with 3,3′ diaminobenzidine (brown). After counterstaining with hematoxylin, samples were dehydrated and mounted. A negative control without primary antibody was included in each staining.
Double staining for NF-κB and PECAM-1 (CD31), a specific endothelial surface protein, was performed as described previously (36). In brief, after incubation with rabbit anti-human NF-κB, an HRP-conjugated goat-anti-rabbit antibody (Dako) was used as second antibody. Detection reaction was developed with 3,3′ diaminobenzidine followed by 10 min of incubation in 0.3% hydrogen peroxide in methanol. In the second step, the antibody against CD31 (diluted 1:20; clone M 0823; Dako) was mounted and detected with a goat anti-mouse HRP antibody (Dako). The second color reaction was developed with the HistoGreen substrate kit (green; Linaris, Wertheim, Germany). No counterstaining was performed.
Analysis of immunohistochemical staining.
For HSP70 and NF-κB, a total of 150 capillaries per sample in placental terminal villi were analyzed. The percentage of positive capillaries was recorded at a magnification of ×200. Capillaries that were stained along the circumference were recorded as positive. VEGF, VEGF-R1, VEGF-R2, TIE-2, and HIF-1α were expressed on all endothelial cells (22,37–39). Therefore, the intensity of staining was measured with a three-step semiquantitative scale (magnification ×200) as weak (+1), moderate (+2), or strong (+3) (40). The average for each sample was calculated out of five analyzed areas that were chosen at random.
Statistical analysis.
Differences between groups were tested with the Kruskal-Wallis H test (for gestational age, birth weight, umbilical cord artery pH, and Apgar score after 5 min), the χ2 test (for gender), and the Mann-Whitney U test for all other comparisons. A p ≤ 0.05 was considered significant.
RESULTS
Fetal capillaries were stressed by chorioamnionitis and funisitis.
The percentage of HSP70-positive capillaries was higher in placenta tissue of the chorioamnionitis group (median 24%; 95% CI 20–27%) and the funisitis group (median 25%; 95% CI 22–27%) in comparison with the control group (median 3%; 95% CI 2–7%; p < 0.05). The result of each analyzed sample is shown in Fig. 1. The percentage of HSP70-positive cells was not significantly different between the funisitis group and the chorioamnionitis group.
Chorioamnionitis and funisitis activated NF-κB in endothelial cells.
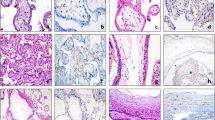
The percentage of NF-κB–positive capillaries was increased in the chorioamnionitis group (median 40%; 95% CI 33–48%) versus the control group (median 16%; 95% CI 9–20%; p < 0.05). The percentage in the funisitis group (median 47%; 95% CI 41–53%) was increased in comparison with the control group but not different from the chorioamnionitis group (p < 0.05). The result of each analyzed sample is summarized in Fig. 2. Representative samples are shown in Fig. 3A and B. The staining was localized to the inflammatory cells in the capillary lumen and to the nuclei of the endothelial cells. A double-staining PECAM-1 (CD31), which is an endothelial marker, and NF-κB were performed to demonstrate the co-localization (Fig. 3C).
Immunohistochemistry. (A and B) NF-κB–positive capillaries were detected in placenta tissue with chorioamnionitis but not in controls. (C) Nuclei of endothelial cells were positive for NF-κB in the chorioamnionitis group, which was shown by staining for CD31 (brown) and NF-κB (green). (D and E) VEGF expression was decreased in placenta tissue from chorioamnionitis. Magnification: ×200 in A, B, D, and E; ×1000 in C.
VEGF was decreased in chorioamnionitis and funisitis.
The intensity of VEGF expression in fetal capillaries was decreased in the chorioamnionitis and in the funisitis group. Representative samples are shown in Fig. 3D and E. The intensity of all analyzed samples is shown in Fig. 4. The intensity in the control group (median 2.3; 95% CI 1.9–2.6) was higher than in the chorioamnionitis group (median 0.7; 95% CI 0.4–1.1) or in the funisitis group (median 1.1; 95% CI 0.8–1.4; p < 0.05).
VEGF-R1 and -R2 were decreased in chorioamnionitis and funisitis.
The intensity of expression of VEGF-R1 and -R2 are demonstrated in Fig. 5A and B, respectively. The intensity of VEGF-R1 was decreased in the control group (median 2.6; 95% CI 2.4–2.8; p < 0.05) compared with the chorioamnionitis group (median 0.5; 95% CI 0.4–0.8) and the funisitis group (median 0.7, 95% CI 0.5–0.9; Fig. 5A). Similar effects were seen with the expression of VEGF-R2 (Fig. 5B). The intensity was reduced in the chorioamnionitis group (median 0.5; 95% CI 0.4–0.8) and the funisitis group (median 0.7; 95% CI 0.5–0.9) in comparison with the control group (median 2.6; 95% CI 2.4–2.8; p < 0.05).
TIE-2 was increased in chorioamnionitis and funisitis.
The staining pattern for TIE-2 was different from the patterns seen with VEGF and its receptors. The intensity of expression of TIE-2 was markedly increased in the chorioamnionitis group and in the funisitis group (Fig. 6). The intensity was increased almost 3-fold in the chorioamnionitis group (median 2.4; 95% CI 2.2–2.7) and in the funisitis group (median 2.5; 95% CI 2.2–2.7) over the control group (median 0.8; 95% CI 0.5–1.0; p < 0.05).
HIF-1 α was not changed in chorioamnionitis and funisitis.
There was no difference in the staining pattern and only minor differences in the intensity of the expression of HIF-1α in all three groups: chorioamnionitis group (median 1.6; 95% CI 1.1–1.8), funisitis group (median 1.8; 95% CI 1.6–2.2), and control group (median 1.9; 95% CI 1.5–2.2; Fig. 7).
DISCUSSION
We found in this study that inflammation in the placenta affected the homeostasis of endothelial cells in the placental capillaries. Expression of HSP70 was increased in placentas from patients with funisitis or chorioamnionitis. Proinflammatory gene transcription was activated in endothelial cells. Expression of proangiogenic VEGF and VEGF-R1 and -R2 were decreased in cases of funisitis and chorioamnionitis, whereas TIE-2 expression was increased. The changes reflected the involvement of placental capillaries in the inflammatory process with possible impact on placental function. Remarkably, the changes were similar in the chorioamnionitis and the funisitis groups and do not reflect the idea of funisitis as a progression of chorioamnionitis. However, we focused on angiogenic changes in capillaries of the placental compartment that may not be involved in the progression of chorioamnionitis to funisitis. In contrast, the analysis of adhesion molecules in the umbilical cord arteries and veins in the study of D'Alquen et al. (33) supported the idea of funisitis as a progression of inflammation to large blood vessels in a different compartment.
The impact of inflammatory and angiogenic changes on placental function remain unclear. However, Satosar et al. (41) recently reported the correlation of inflammation of the villi by bacterial or viral infection with neonatal mortality and morbidity. NF-κB can be induced by many different proinflammatory agents, such as Gram-negative and Gram-positive bacteria or proinflammatory cytokines. The effects on NF-κB activation on angiogenesis in the placenta are not well understood. HIF-1α, VEGF, VEGF receptors, and TIE-2 have been detected on trophoblasts and endothelial cells (22,37–39). The mRNA levels of VEGF and VEGF receptors were dependent on gestational age and mode of delivery, but no analysis of chorioamnionitis was included (38). The expression of VEGF was affected by pathologic conditions such as preterm premature rupture of membranes (43), recurrent miscarriages (44), hypoxia/ischemia (45), fetal growth retardation, fetal alcohol syndrome, or diabetes (31). Marvin et al. (42) showed a reduction of VEGF-R2 mRNA in preterm deliveries with infection in comparison with preterm deliveries without infection. Protein was not quantified in that study. The regulation of VEGF and its receptors occurs at transcriptional, translational, and posttranslational levels (46). In our study, protein expression but not mRNA was analyzed. Therefore, the comparison of the results is limited to the lack of information on respective data on protein or mRNA. The reduction of expression of VEGF and VEGF receptors and the increase in TIE-2 in chorioamnionitis may reflect injury of the placental vessels. The angiogenesis may be impaired, which is reflected by a decrease in VEGF and its receptors. The increase in TIE-2 expression may be part of the repair after inflammation-mediated injury in placenta capillaries because TIE-2 mediates vascular stabilization and remodeling (32). This assumption remains speculative because there was no morphologic difference in the analyzed sections.
The expression of HIF-1α was not changed in patients with chorioamnionitis or funisitis compared with the control group. One possible explanation for this finding is that the changes of HIF-1α have been transient in the course of inflammation and infection. The half-life of HIF-1α is extremely short at ∼5 min. Hypoxia and ischemia acutely extend the half-life of HIF-1α by inhibiting protein degradation (46). We have no information on the onset or the duration of inflammation in these patients. The early changes of inflammation may not have been available for analysis in our study. Another possibility is that endothelial VEGF and VEGF-receptor gene expression in chorioamnionitis may be regulated in a HIF-1α–independent manner for example by transmembrane tyrosine kinases such as the epidermal growth factor receptor (47,48).
The implications of the present study may not be limited to the placenta. Recent findings in preterm infants with pulmonary morbidity highlight effects of infection and chorioamnionitis on neonatal organs other than the placenta (49,50). The role of angiogenesis in the development of BPD was shown in lungs of preterm infants who developed BPD (14,15). The expression of VEGF and its receptors was decreased in infants who developed BPD. Therefore, the interaction of alveogenesis and vascular development was impaired in patients with BPD (51). Apparently, the development of BPD can be primed by exposure to prenatal pulmonary inflammation, which can be aggravated by postnatal events (8,52,53). Structural changes by apoptosis of parenchymal cells were induced in the course of injury and inflammation in the airways and lungs (54). Apparently, alveolar inflammation affects endothelial function and angiogenesis (8). This concept was demonstrated recently by reduced expression of endothelial cell proteins in the lungs of lamb fetuses within days after alveolar inflammation in an endotoxin-induced chorioamnionitis model with little systemic inflammation (55,56). However, lung function can be affected by vascular proinflammatory stimuli as well (51). Our study supports the concept that angiogenesis-related genes may be interesting new therapeutic targets for the prevention and treatment of chorioamnionitis-associated neonatal morbidity and mortality (51).
Our study showed that fetal capillaries are stressed by chorioamnionitis. The expression of VEGF and VEGF-R1 and -R2 was reduced, whereas TIE-2 was increased. The importance of this disturbance of angiogenic homeostasis for placental function and neonatal morbidity remains to be determined. Our findings suggest for the first time that impaired fetal angiogenesis in the placenta might be a pathogenetic mechanism contributing to increased morbidity and mortality of fetuses and newborns who are exposed to chorioamnionitis and funisitis.
Abbreviations
- BPD:
-
bronchopulmonary dysplasia
- CI:
-
confidence interval
- HIF-1α:
-
hypoxia-inducible factor-1α
- HRP:
-
horseradish peroxidase
- HSP70:
-
heat-shock protein 70
- IL:
-
interleukin
- NF-κB:
-
nuclear factor-κB
- TIE-2:
-
tyrosine kinase with immunoglobulin and epidermal growth factor homology domains-2
- VEGF:
-
vascular endothelial growth factor
- VEGF-R:
-
vascular endothelial growth factor receptor
References
Lahra MM, Jeffery HE 2004 A fetal response to chorioamnionitis is associated with early survival after preterm birth. Am J Obstet Gynecol 190: 147–151
Goldenberg RL, Hauth JC, Andrews WW 2000 Intrauterine infection and preterm delivery. N Engl J Med 342: 1500–1507
Sampson JE, Theve RP, Blatman RN, Shipp TD, Bianchi DW, Ward BE, Jack RM 1997 Fetal origin of amniotic fluid polymorphonuclear leukocytes. Am J Obstet Gynecol 176: 77–81
Yoon BH, Romero R, Park JS, Kim M, Oh SY, Kim CJ, Jun JK 2000 The relationship among inflammatory lesions of the umbilical cord (funisitis), umbilical cord plasma interleukin 6 concentration, amniotic fluid infection, and neonatal sepsis. Am J Obstet Gynecol 183: 1124–1129
Watterberg KL, Scott SM, Naeye RL 1997 Chorioamnionitis, cortisol, and acute lung disease in very low birth weight infants. Pediatrics 99: E6
Shimoya K, Taniguchi T, Matsuzaki N, Moriyama A, Murata Y, Kitajima H, Fujimura M, Nakayama M 2000 Chorioamnionitis decreased incidence of respiratory distress syndrome by elevating fetal interleukin-6 serum concentration. Hum Reprod 15: 2234–2240
Costeloe K, Hennessy E, Gibson AT, Marlow N, Wilkinson AR 2000 The EPICure study: outcomes to discharge from hospital for infants born at the threshold of viability. Pediatrics 106: 659–671
Speer CP 2003 Inflammation and bronchopulmonary dysplasia. Semin Neonatol 8: 29–38
Smith LE 2003 Pathogenesis of retinopathy of prematurity. Semin Neonatol 8: 469–473
DiSalvo D, The Developmental Epidemiology Network Investigators 1998 The correlation between placental pathology and intraventricular hemorrhage in the preterm infant. Pediatr Res 43: 9–15
Leviton A, Paneth N, Reuss ML, Susser M, Allred EN, Dammann O, Kuban K, Van Marter LJ, Pagano M, Hegyi T, Hiatt M, Sanocka U, Shahrivar F, Abiri M, Disalvo D, Doubilet P, Kairam R, Kazam E, Kirpekar M, Rosenfeld D, Schonfeld S, Share J, Collins M, Genest D, Shen-Schwarz S et al. 1999 Maternal infection, fetal inflammatory response, and brain damage in very low birth weight infants. Developmental Epidemiology Network Investigators. Pediatr Res 46: 566–575
Nelson KB, Dambrosia JM, Grether JK, Phillips TM 1998 Neonatal cytokines and coagulation factors in children with cerebral palsy. Ann Neurol 44: 665–675
Yoon BH, Romero R, Park JS, Kim CJ, Kim SH, Choi JH, Han TR 2000 Fetal exposure to an intra-amniotic inflammation and the development of cerebral palsy at the age of three years. Am J Obstet Gynecol 182: 675–681
Lassus P, Turanlahti M, Heikkila P, Andersson LC, Nupponen I, Sarnesto A, Andersson S 2001 Pulmonary vascular endothelial growth factor and Flt-1 in fetuses, in acute and chronic lung disease, and in persistent pulmonary hypertension of the newborn. Am J Respir Crit Care Med 164: 1981–1987
Bhatt AJ, Pryhuber GS, Huyck H, Watkins RH, Metlay LA, Maniscalco WM 2001 Disrupted pulmonary vasculature and decreased vascular endothelial growth factor, Flt-1, and TIE-2 in human infants dying with bronchopulmonary dysplasia. Am J Respir Crit Care Med 164: 1971–1980
Demir R, Kaufmann P, Castellucci M, Erbengi T, Kotowski A 1989 Fetal vasculogenesis and angiogenesis in human placental villi. Acta Anat (Basel) 136: 190–203
Bergers G, Benjamin LE 2003 Tumorigenesis and the angiogenic switch. Nat Rev Cancer 3: 401–410
Georgiades P, Ferguson-Smith AC, Burton GJ 2002 Comparative developmental anatomy of the murine and human definitive placentae. Placenta 23: 3–19
Matsuda T, Nakajima T, Hattori S, Hanatani K, Fukazawa Y, Kobayashi K, Fujimoto S 1997 Necrotizing funisitis: clinical significance and association with chronic lung disease in premature infants. Am J Obstet Gynecol 177: 1402–1407
Morimoto RI, Kroeger PE, Cotto JJ 1996 The transcriptional regulation of heat shock genes: a plethora of heat shock factors and regulatory conditions. In: Feige U, Morimoto RI, Yahara I, Polla B (eds) Stress-Inducible Cellular Responses. Birkhäuser Verlag, Basel, pp 38–55
Kelly BD, Hackett SF, Hirota K, Oshima Y, Cai Z, Berg-Dixon S, Rowan A, Yan Z, Campochiaro PA, Semenza GL 2003 Cell type-specific regulation of angiogenic growth factor gene expression and induction of angiogenesis in nonischemic tissue by a constitutively active form of hypoxia-inducible factor 1. Circ Res 93: 1074–1081
Rajakumar A, Conrad KP 2000 Expression, ontogeny, and regulation of hypoxia-inducible transcription factors in the human placenta. Biol Reprod 63: 559–569
Shah M, Stanek J, Handwerger S 1998 Differential localization of heat shock proteins 90, 70, 60 and 27 in human decidua and placenta during pregnancy. Histochem J 30: 509–518
Feinstein DL, Galea E, Aquino DA, Li GC, Xu H, Reis DJ 1996 Heat shock protein 70 suppresses astroglial-inducible nitric-oxide synthase expression by decreasing NFκB activation. J Biol Chem 271: 17724–17732
Baur FM, Brenner B, Goetze-Speer B, Neu S, Speer CP 1998 Natural porcine surfactant (Curosurf) down-regulates mRNA of tumor necrosis factor-α (TNF-α) and TNF-α type II receptor in lipopolysaccharide-stimulated monocytes. Pediatr Res 44: 32–36
Dikov MM, Oyama T, Cheng P, Takahashi T, Takahashi K, Sepetavec T, Edwards B, Adachi Y, Nadaf S, Daniel T, Gabrilovich DI, Carbone DP 2001 Vascular endothelial growth factor effects on nuclear factor-κB activation in hematopoietic progenitor cells. Cancer Res 61: 2015–2021
Cao L, Liu C, Cai B, Jia X, Kang L, Speer CP, Sun B 2004 Nuclear factor-kappa B expression in alveolar macrophages of mechanically ventilated neonates with respiratory distress syndrome. Biol Neonate 86: 116–123
Ferrara N, Gerber HP, LeCouter J 2003 The biology of VEGF and its receptors. Nat Med 9: 669–676
Okada F, Rak JW, Croix BS, Lieubeau B, Kaya M, Roncari L, Shirasawa S, Sasazuki T, Kerbel RS 1998 Impact of oncogenes in tumor angiogenesis: mutant K-ras up-regulation of vascular endothelial growth factor/vascular permeability factor is necessary, but not sufficient for tumorigenicity of human colorectal carcinoma cells. Proc Natl Acad Sci USA 95: 3609–3614
Zachary I 2003 VEGF signalling: integration and multi-tasking in endothelial cell biology. Biochem Soc Trans 31: 1171–1177
Helske S, Vuorela P, Carpen O, Hornig C, Weich H, Halmesmaki E 2001 Expression of vascular endothelial growth factor receptors 1, 2 and 3 in placentas from normal and complicated pregnancies. Mol Hum Reprod 7: 205–210
Sato TN, Tozawa Y, Deutsch U, Wolburg-Buchholz K, Fujiwara Y, Gendron-Maguire M, Gridley T, Wolburg H, Risau W, Qin Y 1995 Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature 376: 70–74
d'Alquen D, Kramer BW, Seidenspinner S, Marx A, Berg D, Groneck P, Speer CP 2005 Activation of umbilical cord endothelial cells and fetal inflammatory response in preterm infants with chorioamnionitis and funisitis. Pediatr Res 57: 263–269
Naeye RL 1987 Functionally important disorders of the placenta, umbilical cord, and fetal membranes. Hum Pathol 18: 680–691
Salafia CM, Weigl C, Silberman L 1989 The prevalence and distribution of acute placental inflammation in uncomplicated term pregnancies. Obstet Gynecol 73: 383–389
Kammerer U, Eggert AO, Kapp M, McLellan AD, Geijtenbeek TB, Dietl J, van Kooyk Y, Kampgen E 2003 Unique appearance of proliferating antigen-presenting cells expressing DC-SIGN (CD209) in the decidua of early human pregnancy. Am J Pathol 162: 887–896
Banks RE, Forbes MA, Searles J, Pappin D, Canas B, Rahman D, Kaufmann S, Walters CE, Jackson A, Eves P, Linton G, Keen J, Walker JJ, Selby PJ 1998 Evidence for the existence of a novel pregnancy-associated soluble variant of the vascular endothelial growth factor receptor, Flt-1. Mol Hum Reprod 4: 377–386
Ahmed A, Li XF, Dunk C, Whittle MJ, Rushton DI, Rollason T 1995 Colocalisation of vascular endothelial growth factor and its Flt-1 receptor in human placenta. Growth Factors 12: 235–243
Goldman-Wohl DS, Ariel I, Greenfield C, Lavy Y, Yagel S 2000 Tie-2 and angiopoietin-2 expression at the fetal-maternal interface: a receptor ligand model for vascular remodelling. Mol Hum Reprod 6: 81–87
Kramer BW, Kramer S, Ikegami M, Jobe AH 2002 Injury, inflammation, and remodeling in fetal sheep lung after intra-amniotic endotoxin. Am J Physiol 283: L452–L459
Satosar A, Ramirez NC, Bartholomew D, Davis J, Nuovo GJ 2004 Histologic correlates of viral and bacterial infection of the placenta associated with severe morbidity and mortality in the newborn. Hum Pathol 35: 536–545
Marvin KW, Keelan JA, Eykholt RL, Sato TA, Mitchell MD 2002 Expression of angiogenic and neurotrophic factors in the human amnion and choriodecidua. Am J Obstet Gynecol 187: 728–734
Daneshmand SS, Chmait RH, Moore TR, Bogic L 2002 Preterm premature rupture of membranes: vascular endothelial growth factor and its association with histologic chorioamnionitis. Am J Obstet Gynecol 187: 1131–1136
Vuorela P, Carpen O, Tulppala M, Halmesmaki E 2000 VEGF, its receptors and the tie receptors in recurrent miscarriage. Mol Hum Reprod 6: 276–282
Kumazaki K, Nakayama M, Suehara N, Wada Y 2002 Expression of vascular endothelial growth factor, placental growth factor, and their receptors Flt-1 and KDR in human placenta under pathologic conditions. Hum Pathol 33: 1069–1077
Xie K, Wei D, Shi Q, Huang S 2004 Constitutive and inducible expression and regulation of vascular endothelial growth factor. Cytokine Growth Factor Rev 15: 297–324
Slomiany MG, Rosenzweig SA 2004 IGF-1-induced VEGF and IGFBP-3 secretion correlates with increased HIF-1 alpha expression and activity in retinal pigment epithelial cell line D407. Invest Ophthalmol Vis Sci 45: 2838–2847
Petit AM, Rak J, Hung MC, Rockwell P, Goldstein N, Fendly B, Kerbel RS 1997 Neutralizing antibodies against epidermal growth factor and ErbB-2/neu receptor tyrosine kinases down-regulate vascular endothelial growth factor production by tumor cells in vitro and in vivo: angiogenic implications for signal transduction therapy of solid tumors. Am J Pathol 151: 1523–1530
Schmidt B, Cao L, Mackensen-Haen S, Kendziorra H, Klingel K, Speer CP 2001 Chorioamnionitis and inflammation of the fetal lung. Am J Obstet Gynecol 185: 173–177
May M, Marx A, Seidenspinner S, Speer CP 2004 Apoptosis and proliferation in lungs of human fetuses exposed to chorioamnionitis. Histopathology 45: 283–290
Abman SH 2001 Bronchopulmonary dysplasia: “a vascular hypothesis.”. Am J Respir Crit Care Med 164: 1755–1756
Speer CP 2001 New insights into the pathogenesis of pulmonary inflammation in preterm infants. Biol Neonate 79: 205–209
Speer CP, Ruess D, Harms K, Herting E, Gefeller O 1993 Neutrophil elastase and acute pulmonary damage in neonates with severe respiratory distress syndrome. Pediatrics 91: 794–799
May M, Strobel P, Preisshofen T, Seidenspinner S, Marx A, Speer CP 2004 Apoptosis and proliferation in lungs of ventilated and oxygen-treated preterm infants. Eur Respir J 23: 113–121
Kallapur SG, Bachurski CJ, Le Cras TD, Joshi SN, Ikegami M, Jobe AH 2004 Vascular changes after intra-amniotic endotoxin in preterm lamb lungs. Am J Physiol 287: L1178–L1185
Kramer BW, Moss TJ, Willet KE, Newnham JP, Sly PD, Kallapur SG, Ikegami M, Jobe AH 2001 Dose and time response after Intramniotic endotoxin in preterm lambs. Am J Respir Crit Med 164: 982–988
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was supported by a grant of the Interdisciplinary Center for Clinical Research (A-27) of the University of Würzburg, Germany.
Rights and permissions
About this article
Cite this article
Kramer, B., Kaemmerer, U., Kapp, M. et al. Decreased Expression of Angiogenic Factors in Placentas with Chorioamnionitis after Preterm Birth. Pediatr Res 58, 607–612 (2005). https://doi.org/10.1203/01.PDR.0000175641.39056.7A
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/01.PDR.0000175641.39056.7A
This article is cited by
-
Role of Transcription Factors in the Management of Preterm Birth: Impact on Future Treatment Strategies
Reproductive Sciences (2023)
-
Autism spectrum disorders in extremely preterm infants and placental pathology findings: a matched case–control study
Pediatric Research (2021)
-
Placental origins of neonatal diseases: toward a precision medicine approach
Pediatric Research (2021)
-
Expression of tumor necrosis factor-alpha and vascular endothelial growth factor in different zones of fetal membranes: a possible relation to onset of labor
Journal of Molecular Histology (2014)
-
Chorioamnionitis, respiratory distress syndrome and bronchopulmonary dysplasia in extremely low birth weight infants
Journal of Perinatology (2011)