Abstract
Exposure to preconditioning (PC) hypoxia 24 h before a severe hypoxic-ischemic (HI) insult reduces development of injury in the immature brain. Several protective regimens have proved effective in the short-term but not in the long-term perspective. The aim of the present study, therefore, was to evaluate the PC effect on long-term morphologic and neurologic outcome in the developing brain. Six-day-old rats were subjected to hypoxia (36°C, 8.0% O2; PC/HI group) and sham controls to normoxia (36°C; HI group) for 3 h. Twenty-four hours later, all rats were exposed to cerebral HI produced by unilateral carotid artery occlusion combined with 1 h, 15 min of hypoxia (36°C, 7.7% O2). A cylinder test was used to evaluate forelimb asymmetry to determine sensorimotor function at 4, 6, and 8 wk of age. Spatial/cognitive ability was assessed by Morris water maze trials at 7 wk of recovery. Neuropathologic analysis was performed 8 wk after insult. Brain damage was reduced (p < 0.0001) in PC/HI (45.0 ± 11.1 mm3) in comparison with HI (159.3 ± 12.2 mm3) rats. A bias for using the ipsilateral forelimb in wall movements was observed in the cylinder test in HI compared with PC/HI rats at 4 (p < 0.001), 6 (p < 0.01), and 8 (p < 0.0001) wk of age. Results of the Morris water maze test revealed differences (p < 0.0001) in average path length between groups on the third and fourth day of trials. Hypoxic PC before HI reduced brain injury by 72% at 8 wk after the insult and provided long-term improvement of sensorimotor and spatial/cognitive functions.
Similar content being viewed by others
Main
Hypoxic-ischemic (HI) brain injury during the perinatal period is a major cause of lifelong disability (1,2). The development of effective therapeutic and/or preventive strategies to treat these pathologic events relies on a better understanding of the critical pathophysiologic events that lead to HI brain injury.
In 1986, Murry et al. (3) reported that the extent of myocardial infarction resulting from a sustained coronary occlusion was diminished when the heart had been subjected to brief periods of sublethal ischemia. The protection elicited by a previous sublethal intervention that renders the tissue less sensitive to a subsequent insult has been termed preconditioning (PC). PC can be induced in the CNS through a variety of exposures, for example, seizures (4), hypoxia (5), lipopolysaccharide (6), and mitochondrial toxins (7).
A PC model has been developed for the immature brain, whereby exposure to 8% hypoxia 24 h before severe HI reduces injury by 70–100% (5,8,9). Findings of protection have been demonstrated from 1 to 3 wk after insult. Recent studies indicate, however, that development of injury in the immature brain after HI may be delayed for up to 8 wk (10), and in some cases, the salutary effects of PC in the adult (11) are partly lost after long recovery periods after the severe insult. A number of treatments after HI in immature animals have also been shown to delay injury rather than provide a permanent protection (12). Furthermore, deficits in spatial/cognitive and sensorimotor functions sometimes occur despite no or subtle brain injury, implicating the need for sophisticated functional assessments as a complement to evaluation of lesion size (13). Our aim, therefore, was to further characterize PC in the immature brain and to determine to what extent hypoxic PC provides long-lasting behavioral and histologic protection.
METHODS
Sprague-Dawley rats (obtained from Charles River, Sulzfeld, Germany) were bred at the local animal care facility (EBM, Göteborg University, Göteborg, Sweden) and housed in accordance with guidelines of the Animal Committee of the University of Göteborg. The animal experiments were approved by the local Ethical Committee of Göteborg (no. 146-03).
Hypoxic PC.
On postnatal day 6 (PND6), pups were exposed to a PC interval of hypoxia (PC/HI group; n = 67). Rats were placed in a chamber perfused with a humidified gas mixture (8.0% oxygen in nitrogen) at 36°C for 3 h. Simultaneously, littermate sham control rats were separated from the dam and exposed to normoxia 36°C for 3 h (HI group; n = 63). Additional naïve controls (n = 22) remained with their dams and were untreated. Pups were randomly grouped from a total of 15 litters.
HI insult.
Twenty-four hours after the hypoxic PC exposure, pups were subjected to an HI insult in accordance with preparations previously described by Rice et al. (14). Rats were anesthetized with enflurane (3% for induction and 1.5% for maintenance) in nitrous oxide/oxygen (1:1). The left common carotid artery was dissected and cut between ligatures of prolene sutures (6-0). Furthermore, the wound was closed and infiltrated with a local anesthetic (Xylocaine, 40 mg/mL). The surgical procedure was followed by 1 h of recovery. HI was induced by exposure to a humidified gas mixture (7.7% oxygen in nitrogen) at 36°C for 1 h, 15 min. The pups were allowed to adjust to the temperature/humidity of the chamber for 10 min before and 10 min after hypoxia. After HI, the pups were returned to their dam. Naïve controls remained untreated and were not separated from their dam.
Evaluation of PC on short-term outcome.
Pups (PC/HI n = 37, HI n = 33) were killed at PND14. Rats were deeply anesthetized (Pentothal Natrium, 50 mg/mL) and perfused intracardially with 0.9% NaCl followed by 5% paraformaldehyde. Brains were macroscopically scored for neuropathologic outcome according to Bona et al. (15).
Cylinder test.
Forelimb use bias was analyzed by videotaping movements of each rat during exploratory activity in a transparent cylinder (16). The size of the cylinder allowed free movements but was small enough to encourage rearing and wall exploration. Its height prevented the rat from reaching the top edge. A mirror was placed at an angle behind the cylinder to allow correct analysis of movements. A mirror was placed in an angle behind the cylinder in order to allow correct analysis of movements as the rat was faced away from the camera. Depending on the activity of each rat. the duration of trials varied from 3 to 7 min. Scoring of each trial sequence consisted of independent versus combined use of the forelimbs during vertical exploration of the walls and when landing as described previously (16,17). Results were expressed as 1) the percentage of use of the nonimpaired forelimb relative to the total number of limb-use movements, 2) the percentage of use of the impaired forelimb relative to the total number of limb-use movements, and 3) the percentage of co-use of both limbs relative to the total number of limb-use movements. The percentage of the use of impaired forelimb was then subtracted from the percentage of the nonimpaired forelimb for exploration and landing. These two scores (wall and landing) were averaged together for a single limb-use asymmetry score that corrected for variability in the number of wall versus landing movements. Exclusion of trials occurred in cases of inactivity, where the rat performed <5 landings and <10 wall movements during a test session. The forelimb use asymmetry test was carried out at 4, 6, and 8 wk of age.
Morris water maze test.
Each rat performed a series of four trials at each occasion for 4 consecutive days. In a large water-filled tank (160 cm in diameter), rats were monitored by a video-tracking system while searching for a submerged platform (Smart_DT version 2.00, Panlab). The water was kept at room temperature and made opaque by adding dry milk powder. Each rat was given a maximum of 40 s to find the hidden platform. When the rat failed to find the platform, it was designated as having a 40-s latency, physically guided to the platform, and allowed to remain there for an additional 15 s. Parameters monitored were the latency to find the platform, the swimming distance, and the pattern of the swimming track. The Morris water maze test was performed at 8 wk of age.
Evaluation of PC on long-term outcome.
Rats were tested for their functional ability 4, 6, and 8 wk after HI. At 9 wk of age, they were killed through perfusion fixation as described above. Brains were subjected to macroscopic scoring as previously described. Thereafter, the brains were dehydrated, embedded in paraffin, and sectioned into 5-μm-thick coronal sections evenly distributed throughout the brain (every 100th section), resulting in approximately 15 anteroposterior levels. Sections were stained with thionin/acid fuchsin (18) for detailed morphologic analysis.
Neuropathologic analysis.
Brain tissue deficit was calculated on sections that were stained with thionin/acid fuchsin using the Olympus Micro Image analysis software system, version 4.0 (Olympus Optical, Tokyo, Japan). Tissue loss was measured at each anatomical level by subtracting the area of the ipsilateral hemisphere from the area of the contralateral hemisphere and expressed as percentage of the contralateral hemisphere. The volume of tissue loss was calculated according to the Cavalieri Principle using the formula V = ΣAPt, where V is the total volume, ΣA is the sum of the areas measured, P is the inverse of the section sampling fraction, and t is the section thickness (19).
Regional differences in brain damage were evaluated using a semiquantitative neuropathologic scoring system with slight modifications (20). Briefly, the cortical injury was graded from 0 to 4, that is, from no observable injury (score = 0) to confluent infarction encompassing most of the ipsilateral hemisphere (score = 4). The damage in hippocampus, striatum, and thalamus was assessed regarding both hypotrophy (0–3) and observable cell injury/infarction (0–3), resulting in a neuropathologic score for each brain region (0–6). The total score (0–22) was the sum score for all four regions.
Statistics.
Statistical analyses were performed using two-way ANOVA, and group comparisons were performed using Fisher t test. All data are expressed as means ± sem.
RESULTS
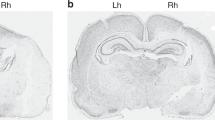
Macroscopic scoring of brains of rats that were evaluated at PND14 revealed a significant reduction of damage in the PC/HI group (0.9 ± 0.2; n = 37) when compared with the HI group (2.7 ± 0.2; n = 33; p < 0.0001). At 8 wk after the insult, PC was similarly protective, with damage in PC/HI rats (0.6 ± 0.2; n = 30) significantly reduced compared with HI rats (2.5 ± 0.2; n = 30; p < 0.0001). No injury was detected in control rats (0 ± 0; n = 22). Microscopic quantitative analysis showed a 72% reduction of tissue volume loss in the PC/HI group (45.0 ± 11.1 mm3; n = 30) compared with rats in the HI group (159.3 ± 12.2 mm3; n = 30; p < 0.0001). The area of brain tissue deficit measured in coronal sections, expressed as percentage of the contralateral hemisphere, was significantly reduced in PC/HI compared with HI rats at every level (Fig. 1A). Regional scoring demonstrated statistically reduced brain lesions in cerebral cortex, hippocampus, striatum, and thalamus in the PC/HI group versus the HI group (Fig. 1B). The total neuropathologic score was significantly lower in PC/HI (4.6 ± 1.2; n = 30) compared with HI (18.2 ± 1.3; n = 30; p < 0.0001) rats.
Neuropathologic results. Brain injury was substantially reduced in the PC/HI group (n = 30) compared with the HI group (n = 30) 8 wk after HI. (A) Brain damage was quantified in 15 coronal sections numbered from anterior to posterior levels. Measurements are expressed as tissue loss in the ipsilateral hemisphere as a percentage of the contralateral hemisphere. Values are given as mean ± sem; ‡p < 0.001 PC/HI vs HI rats. (B) Regional scoring of injury in cerebral cortex, hippocampus, striatum, and thalamus. Values are given as mean ± sem; ‡p < 0.001 for PC vs non-PC HI rats. (C) Typical coronal sections of littermates demonstrating the difference in brain injury of PC/HI (left) compared with HI rats (right).
In the cylinder test (Fig. 2), HI rats showed a pronounced bias toward using the ipsilateral forelimb for wall exploration (38 ± 6%; n = 26) during initial trials at 4 wk of age. In later trials, a persistent tendency of ipsilateral forelimb asymmetry was seen at 6 (37 ± 6%; n = 24) and 8 wk (43 ± 5%; n = 25) of age, respectively. Preconditioned rats improved in unimpaired forelimb bias for wall movements at 4 (14 ± 4%; n = 28; p < 0.001), 6 (13 ± 5%; n = 27; p < 0.01), and 8 wk of age (5 ± 7%; n = 27; p < 0.0001). Controls displayed a less pronounced ipsilateral bias (4 wk: 7 ± 5%, n = 17; 6 wk: 7 ± 5%, n = 14; 8 wk: 0 ± 3%, n = 20).
Cylinder test results. Forelimb asymmetry was attenuated in the PC/HI group compared with the HI control group. Wall movements, landings, and average values are given at 4 (PC/HI n = 28, HI n = 26, control n = 17; A), 6 (PC/HI n = 27, HI n = 24, control n = 14; B) and 8 (PC/HI n = 27, HI n = 25, control n = 20; C) wk of age. Functional asymmetry was assessed by evaluating the bias in usage of impaired vs unimpaired forelimb. Values are given as mean ± sem; ‡p < 0.001; †p < 0.01 vs HI animals.
The average of forelimb use for wall explorations and landings demonstrated a difference in behavioral asymmetry between PC/HI rats (0 ± 2%, p = 0.05; 2 ± 2%, p < 0.01; −2 ± 3%, p < 0.001) in comparison with HI rats (8 ± 4%; 12 ± 3%; 12 ± 4%) at 4, 6, and 8 wk of age, respectively.
The performance in the Morris water maze test demonstrated distinct differences between the groups (Fig. 3). Over the 4 consecutive days of trials, PC/HI and control rats displayed the same behavioral pattern (decrease in mean swim distance), whereas HI rats, on the contrary, did not improve in performance. The mean swim distance and the pattern of swim path were assessed. On the final day of trials, the path length performed by preconditioned rats (6.4 ± 0.6 m; n = 28) and control rats (4.4 ± 0.5 m; n = 20) were significantly shorter than those of HI rats (10.9 ± 0.6 m; n = 29; p < 0.0001). HI rats spent a higher percentage of swim distance in the outer circle of the maze (89 ± 2%; n = 29) in comparison with PC/HI rats (78 ± 2%; n = 28; p < 0.0001) and controls (73 ± 2%; n = 20).
Morris water maze results. Hypoxic PC improved spatial/cognitive performance as evaluated in the Morris water maze. Results demonstrate that the mean swim distance was significantly shorter in PC/HI (n = 28) vs HI rats (n = 29) during 4 consecutive days of trial. There was no significant difference in cognitive ability between PC/HI and non-HI controls (n = 20). Values are given as mean ± sem; ‡p < 0.001; †p < 0.01; *p = 0.05 vs HI animals.
There were no sex differences in the morphologic or the behavioral analyses. Measurements of brain injury volume in preconditioned rats for male (38.4 ± 14.2 mm3; n = 18) and female rats (55.0 ± 18.1 mm3; n = 12; p < 0.4751) were not statistically different. Also in the HI group, brain injury was similar in male (165.5 ± 17.5 mm3; n = 13) and female rats (154.6 ± 17.2 mm3; n = 17; p < 0.6683).
DISCUSSION
In the present study we found that hypoxic PC conferred a reduction in neonatal HI brain damage up to 8 wk after the insult. The difference between groups was in accordance with our findings of short-term protection found in brains that were evaluated macroscopically at 1 wk after insult and in agreement with previously published data on short-term outcome (5,9). It is interesting that histologic findings correlated closely to sensorimotor and spatial/cognitive performance.
Previous studies on hypoxic PC in immature rats were based on histologic (and not functional) assessments. In most of these studies, brain injury was estimated at 1 wk (or shorter) after HI (5,9,21–24) except for one study that reported longer survival (30 d) (8). It is well known that injury often develops over a protracted period of weeks or even months, which can influence the efficacy of treatments. For example, postischemic hypothermia provided significant protection when cell loss was evaluated at 3 and 7 d but not when the morphologic assessment was performed 2 mo after ischemia in adult rats (25). Similar results were obtained in adult gerbils that were subjected to a short period of PC ischemia before severe ischemia (11). They found that 81% of cells survived at 10 d, but when histologic assessment was done at 30 d after the insult, cell survival had declined to 53% (11). It was found recently that development of brain injury could also be delayed after HI induced in 7-d-old rat pups (10). A marked progressive atrophy occurred between 2 and 8 wk after the insult (10). It has also been shown that a short period of postinsult hypothermia provided protection in the short-term (1 wk) but not in the long-term (4 wk) perspective (12). Collectively, these studies illustrate the need to study long-term effects of potential neuroprotective strategies. We now have shown conclusively that the degree of the long-term neuroprotective effects of PC are similar to the short-term protection found in brains that were evaluated macroscopically at 1 wk postinsult and also agree with previously published data on short-term outcome (5,9)
In most animal studies in which neuroprotective interventions are evaluated, histologic outcome after a few days of survival is used as the only outcome measure (13). Measurement of volume tissue loss or assessment of cell loss does not reliably detect more subtle forms of neuronal death and dysfunction that arise from injury to nonhomogeneous cell populations. For example, PC provided a nearly complete protection in the CA1 of the hippocampus 10 d after an ischemic insult, but animals exhibited marked deficits in an open field test of habituation as well as reduced dendritic field potentials, suggesting that these surviving cells were functionally impaired (26). Recently, it was demonstrated that exposure to an enriched environment significantly improved functional outcome despite increased cell death (27), offering another example of a poor correlation between morphologic and neurologic outcome. These reports further emphasize the importance of performing assessments of neurologic functions as a complement to morphologic outcome measures.
In our study, the cylinder test was performed at different time points to determine sensorimotor ability at different ages after HI. Our results revealed better preservation of limb use symmetry in PC/HI rats in comparison with HI rats. In previous publications, the cylinder test has been used in established models of unilateral stroke, Parkinson's disease, and spinal cord injury (16). Hua et al. (28) used the cylinder test to determine the degree of recovery after intracerebral hemorrhage in rats after pharmacologic interventions, and the test also proved useful in the evaluation of the neuroprotective effect of previous forced limb use after 6-hydroxydopamine administration (29). Measurement of asymmetry seems to be well suited for evaluating functional consequences after unilateral brain injury. It is a simple, sensitive, and reliable test that allows the observer to study behavior under unforced conditions (16).
Previously, diverse results of sensorimotor tests have been reported in the immature HI model. Using a battery of tests, Bona et al. (30) found impairments in sensorimotor ability at 5–6 wk after HI. Similar findings were reported by Jansen et al. (31) in somewhat older (up to 9 wk) animals. In contrast, results of Ikeda et al. (32) demonstrated only subtle sensorimotor deficits 4–18 wk after neonatal HI. The divergent results could be explained by the fact that different tests were applied, different measures were taken to reduce stress, the number of test sessions used were different, and there was varying extent of brain lesion and age of the animals (30–32). For obtaining reproducible results, it seems critical to reduce stressful stimuli. From our experience, evaluation of sensorimotor function in freely moving animals that is achievable with the cylinder test is advantageous compared with tests that require handling, such as in the grip-traction test, the foot-fault test, the postural reflex test, and the limb-placing test (30).
The Morris water maze task examines hippocampal function and is a well-established test for permanent spatial learning capabilities and reference memory (33). Several studies have applied the test after hippocampal damage in neonatal animals. Ikeda et al. (32) detected long-term learning disabilities at 16 wk after neonatal HI in comparison with controls. Altemus et al. (34) also found spatial memory deficits in animals with bilateral hippocampal damage at various ages up to adulthood. Despite compensation by the contralateral hemisphere, performance in the Morris water maze correlated closely to the degree of atrophy in our study, which agrees with results by Altemus et al. (34), who found a close correlation between spatial/cognitive ability and bilateral hippocampal injury. Similar to control animals, during the course of trials, PC/HI rats significantly improved in orientation and memory function. No such improvement was seen in HI animals, which further amplified the differences between groups.
CONCLUSION
In summary, we found that hypoxic PC provides a substantial long-term reduction in brain injury in rats subjected to neonatal HI. The structural protection was accompanied by significant improvement of sensorimotor as well as spatial/cognitive abilities.
Abbreviations
- HI:
-
hypoxia-ischemia
- PC:
-
preconditioning
- PND:
-
postnatal day
References
Hagberg B, Hagberg G, Beckung E, Uvebrant P 2001 Changing panorama of cerebral palsy in Sweden. VIII. Prevalence and origin in the birth year period 1991–94. Acta Paediatr 90: 71–277
Levene ML, Kornberg J, Williams TH 1985 The incidence and severity of post-asphyxial encephalopathy in full-term infants. Early Hum Dev 11: 1–26
Murry CE, Jennings RB, Reimer KA 1986 Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 74: 124–1136
Lowenstein DH, Simon RP, Sharp FR 1990 The pattern of 72-kDa heat shock protein-like immunoreactivity in the rat brain following flurothyl-induced status epilepticus. Brain Res 531: 73–182
Gidday JM, Fitzgibbons JC, Shah AR, Park TS 1994 Neuroprotection from ischemic brain injury by hypoxic preconditioning in the neonatal rat. Neurosci Lett 168: 21–224
Zimmermann C, Ginis I, Furuya K, Klimanis D, Ruetzler C, Spatz M, Hallenbeck JM 2001 Lipopolysaccharide-induced ischemic tolerance is associated with increased levels of ceramide in brain and in plasma. Brain Res 895: 9–65
Wiegand F, Liao W, Busch C, Castell S, Knapp F, Lindauer U, Megow D, Meisel A, Redetzky A, Ruscher K, Trendelenburg G, Victorov I, Riepe M, Diener HC, Dirnagl U 1999 Respiratory chain inhibition induces tolerance to focal cerebral ischemia. J Cereb Blood Flow Metab 19: 229–1237
Vannucci RC, Towfighi J, Vannucci SJ 1998 Hypoxic preconditioning and hypoxic-ischemic brain damage in the immature rat: pathologic and metabolic correlates. J Neurochem 71: 215–1220
Gidday JM, Shah AR, Maceren RG, Wang Q, Pelligrino DA, Holtzman DM, Park TS 1999 Nitric oxide mediates cerebral ischemic tolerance in a neonatal rat model of hypoxic preconditioning. J Cereb Blood Flow Metab 19: 31–340
Geddes R, Vannucci RC, Vannucci SJ 2001 Delayed cerebral atrophy following moderate hypoxia-ischemia in the immature rat. Dev Neurosci 23: 80–185
Corbett D, Crooks P 1997 Ischemic preconditioning: a long term survival study using behavioural and histological endpoints. Brain Res 760: 29–136
Trescher WH, Ishiwa S, Johnston MV 1997 Brief post-hypoxic-ischemic hypothermia markedly delays neonatal brain injury. Brain Dev 19: 26–338
Corbett D, Nurse S 1998 The problem of assessing effective neuroprotection in experimental cerebral ischemia. Prog Neurobiol 54: 31–548
Rice JE 3rd, Vannucci RC, Brierley JB 1981 The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol 9: 31–141
Bona E, Aden U, Gilland E, Fredholm BB, Hagberg H 1997 Neonatal cerebral hypoxia-ischemia: the effect of adenosine receptor antagonists. Neuropharmacology 36: 327–1338
Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST 2000 CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology 39: 77–787
Tillerson JL, Cohen AD, Philhower J, Miller GW, Zigmond MJ, Schallert T 2001 Forced limb-use effects on the behavioral and neurochemical effects of 6-hydroxydopamine. J Neurosci 21: 427–4435
Smith ML, Auer RN, Siesjo BK 1984 The density and distribution of ischemic brain injury in the rat following 2–10 min of forebrain ischemia. Acta Neuropathol (Berl) 64: 19–332
Mallard EC, Rehn A, Rees S, Tolcos M, Copolov D 1999 Ventriculomegaly and reduced hippocampal volume following intrauterine growth-restriction: implications for the aetiology of schizophrenia. Schizophr Res 40: 1–21
Hedtjarn M, Leverin AL, Eriksson K, Blomgren K, Mallard C, Hagberg H 2002 Interleukin-18 involvement in hypoxic-ischemic brain injury. J Neurosci 22: 910–5919
Cantagrel S, Krier C, Ducrocq S, Bodard S, Payen V, Laugier J, Guilloteau D, Chalon S 2003 Hypoxic preconditioning reduces apoptosis in a rat model of immature brain hypoxia-ischaemia. Neurosci Lett 347: 06–110
Ota A, Ikeda T, Abe K, Sameshima H, Xia XY, Xia YX, Ikenoue T 1998 Hypoxic-ischemic tolerance phenomenon observed in neonatal rat brain. Am J Obstet Gynecol 179: 075–1078
Wada T, Kondoh T, Tamaki N 1999 Ischemic “cross” tolerance in hypoxic ischemia of immature rat brain. Brain Res 847: 99–307
Ikeda T, Xia XY, Xia YX, Ikenoue T 1999 Hyperthermic preconditioning prevents blood-brain barrier disruption produced by hypoxia-ischemia in newborn rat. Brain Res Dev Brain Res 117: 3–58
Dietrich WD, Busto R, Alonso O, Globus MY, Ginsberg MD 1993 Intraischemic but not postischemic brain hypothermia protects chronically following global forebrain ischemia in rats. J Cereb Blood Flow Metab 13: 41–549
Dooley P, Corbett D 1998 Competing processes of cell death and recovery of function following ischemic preconditioning. Brain Res 794: 19–126
Farrell R, Evans S, Corbett D 2001 Environmental enrichment enhances recovery of function but exacerbates ischemic cell death. Neuroscience 107: 85–592
Hua Y, Schallert T, Keep RF, Wu J, Hoff JT, Xi G 2002 Behavioral tests after intracerebral hemorrhage in the rat. Stroke 33: 478–2484
Cohen AD, Tillerson JL, Smith AD, Schallert T, Zigmond MJ 2003 Neuroprotective effects of prior limb use in 6-hydroxydopamine-treated rats: possible role of GDNF. J Neurochem 85: 99–305
Bona E, Johansson BB, Hagberg H 1997 Sensorimotor function and neuropathology five to six weeks after hypoxia-ischemia in seven-day-old rats. Pediatr Res 42: 78–683
Jansen EM, Low WC 1996 Long-term effects of neonatal ischemic-hypoxic brain injury on sensorimotor and locomotor tasks in rats. Behav Brain Res 78: 89–194
Ikeda T, Mishima K, Yoshikawa T, Iwasaki K, Fujiwara M, Xia YX, Ikenoue T 2001 Selective and long-term learning impairment following neonatal hypoxic-ischemic brain insult in rats. Behav Brain Res 118: 7–25
D'Hooge R, De Deyn PP 2001 Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev 36: 0–90
Altemus KL, Almli CR 1997 Neonatal hippocampal damage in rats: long-term spatial memory deficits and associations with magnitude of hippocampal damage. Hippocampus 7: 03–415
Acknowledgements
A special thanks to Eva Cambert for excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
The work was supported by grants from the Swedish Medical Research Council (09455), the Wilhelm and Martina Lundgren Foundation, the Göteborg Medical Society, and grants to researchers in the public health service from the Swedish government.
Rights and permissions
About this article
Cite this article
Gustavsson, M., Anderson, M., Mallard, C. et al. Hypoxic Preconditioning Confers Long-Term Reduction of Brain Injury and Improvement of Neurological Ability in Immature Rats. Pediatr Res 57, 305–309 (2005). https://doi.org/10.1203/01.PDR.0000151122.58665.70
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/01.PDR.0000151122.58665.70
This article is cited by
-
Sevoflurane Postconditioning Ameliorates Neuronal Migration Disorder Through Reelin/Dab1 and Improves Long-term Cognition in Neonatal Rats After Hypoxic-Ischemic Injury
Neurotoxicity Research (2021)
-
Placental pathology and neonatal brain MRI in a randomized trial of erythropoietin for hypoxic–ischemic encephalopathy
Pediatric Research (2020)
-
Hypoxic preconditioning protection is eliminated in HIF-1α knockout mice subjected to neonatal hypoxia–ischemia
Pediatric Research (2014)
-
Hypobaric Hypoxia Postconditioning Reduces Brain Damage and Improves Antioxidative Defense in the Model of Birth Asphyxia in 7-Day-Old Rats
Neurochemical Research (2014)
-
Vascular Response to Hypoxic Preconditioning in the Immature Brain
Journal of Cerebral Blood Flow & Metabolism (2007)