Abstract
A kinetic modeling of leucine plasma concentration changes is proposed to describe the plasma leucine reduction rate during continuous extracorporeal removal therapy (CECRT) in neonates with maple syrup urine disease. Data were obtained from seven neonates using a bicompartmental model for the best fitted curve of plasma leucine decrease during CECRT. During the first 3 h, leucine plasma levels decreased according to an exponential curve: [Leu]t = [Leu]i × 0.95 × 10-0.09t where [Leu]t is the leucine plasma level (μmol/L) at time t (h) during CECRT and [Leu]I is the initial plasma level. From h 4 to the end of CECRT, a second exponential curve was observed: [Leu]t = [Leu]i × 0.74 × 10-0.05t. Plasma leucine levels obtained from three other neonates were similar to those predicted by the model. The apparent distribution volumes for leucine that correspond to the two exponential equations obtained were calculated from the leucine mass removal collected in the spent dialysate and ultrafiltrate. The distribution volume was 34 ± 3% of body weight during the first 3 h of CECRT and 72 ± 7% from h 4 to the end of CECRT. These figures are similar to known values for the extracellular water compartment and for total body water in the newborn. The findings suggest that leucine handling during CECRT is similar to that of nonprotein-bound small-molecular-weight solutes such as urea.
Similar content being viewed by others
Main
MSUD is an inherited metabolic disease due to a deficiency in branched chain ketoacid dehydrogenase that leads to the accumulation of BCAA (leucine, isoleucine, and valine) and their keto-acid derivatives (1). Because of this impaired enzyme activity, leucine accumulates in cells and body fluids when the load exceeds leucine metabolism capacity and/or when a increased protein catabolism occurs. Given that renal clearance of BCAA and its derivatives is poor and due to the fact that their acute elevation causes neurologic deterioration, CECRT is indicated in severe cases and/or when nutritional support free of BCAA is not possible (2).
Because assessment of leucine plasma levels by amino acid chromatography is not easily available and is time consuming, the aim of this study was to design a kinetic model that predicts leucine plasma level changes over time during the acute phase of the illness in neonates on CECRT.
METHODS
Patients.
Between January 1991 and April 2000, seven neonates with MSUD presented with a severe acute deterioration of their status. After a 6-h period of conservative management that included correction of dehydration and increased caloric intake, all seven patients presented two of the three following criteria that are associated with a high risk of severe brain damage: comatose state, gastrointestinal intolerance, and leucine plasma levels ≥1700 μM. The patients were referred to pediatric and neonatal intensive care for alternative therapies, in particular CECRT (3). Blood and spent dialysate samples were collected to monitor treatment efficiency. We retrospectively used these biologic analyses to elaborate the parameters involved in leucine kinetic modeling during CECRT (Table 1). Subsequently, between May 2000 and December 2001, three other neonates were treated with the same therapeutic strategy and were retrospectively studied to validate the leucine kinetic modeling proposed (Table 2). Approval to undertake this study was obtained from the Necker Institutional Review board and informed consent was obtained from the parents of all patients included.
Patient management.
Newborns were placed in a radiant warmer and mechanically ventilated and sedated. Continuous monitoring of heart rate, temperature, and transcutaneous oxygen saturation was performed. A hemodialysis catheter (6.5-French dual lumen catheter, Hospal Medical Corporation, East Brunswick, NJ) was inserted into the femoral or internal jugular vein. Blood samples were drawn every 3–6 h to obtain blood gases, hematocrit, and activated clotting time as well as total protein, sodium, potassium, calcium, phosphate, urea, and creatinine levels. Blood amino acid chromatography was performed before CECRT, at least twice daily during its course and just before its cessation.
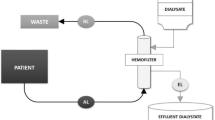
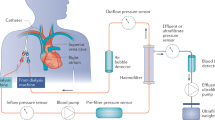
The therapeutic strategy used is described in a previous publication (3) and included resumption of nutritional support 2 h after CECRT initiation. CECRT was performed using a FH 22 Gambro (0.2 m2 polyamide membrane) or Miniflow 10 Hospal (420 cm2 AN 69 membrane) hemofilter connected to the central venous catheter via a dedicated neonatal blood circuit (priming blood volume = 35 mL). Blood flow was continuously monitored (AK-10, Gambro, Colombes, France; BSM 22 PM or Prisma, Hospal, Lyon, France; BM 11, Baxter, Versailles, France). A 4% albumin solution was used to prime the extracorporeal blood circuit and a sodium heparin solution was infused into the arterial line at a rate of 10 IU/kg/h to achieve an activated clotting time 1.5 times that of control. The same sterile nonpyrogenic fluid was used as either dialysate or replacement fluid and was prepared by mixing 5 L of HF 140 (Fresenius Medical Care, Sèvres, France) with 190 mL of 8.4% sodium bicarbonate and 10 mmol of 7.46% KCl to achieve the following composition (in mmol/L): Na+ 140, K+ 3, Ca++ 1.75, Mg++ 0.5, HCO3− 35, Cl− 107 and CH3COO− 3. Replacement fluid was administered in a predilution mode. For the purpose of fluid balance in the study, the volumes of total fresh dialysate, spent dialysate, ultrafiltrate, and replacement fluid were recorded. Flow rate was determined according to hemofilter type, pump ability, and body weight. Twenty milliliter samples drawn from the spent dialysate and the ultrafiltrate bag were frozen for BCAA analysis.
Calculation.
Leucine mass removal, NLeu, is the product of leucine concentration in the ultrafiltrate and spent dialysate and their volume. The integrated clearance (CI) is calculated according to the formula CI = Nleu/(Pm × td), where Pm is the mean leucine plasma concentration and td is the session duration in minutes.
The leucine Vd is the ratio of the leucine mass removal to leucine plasma level decrease during a defined period: Vd = NLeu/(Ct1 − Ct2) where Ct1 and Ct2 are the leucine plasma levels at the start and the end of spent dialysate collection. Distribution volumes were expressed as a percentage of body weight [(Vd × 100)/body weight].
Leucine kinetic modeling.
Based on data obtained from the first seven neonates, kinetic modeling was attempted to describe the time course of plasma leucine levels during CECRT. Least square statistical analysis was used to attain the best fitted curve, which was defined as a linear or exponential curve with a coefficient of determination (r2) > 0.95. Curve fitting was carried out with CA-Cricket Graph III software for the Macintosh (Computer Associates International, Islandia, NY). Given that all seven patients were newborns with a classic form of MSUD and similar weight and age, an integrated leucine clearance within the same range, and no other disease, we included the mean value obtained from all the blood samples drawn to attain the best fitted curve analysis.
RESULTS
Plasma leucine changes during CECRT.
The plasma leucine levels changes during CECRT that were retrospectively obtained from the first seven neonates studied resulted in a best fitted curve suggesting a bicompartmental model (Fig. 1). When this model is applied, plasma leucine levels decrease during the first 3 h according to an exponential curve defined by the equation [Leu]t = [Leu]i × 0.95 × 10-0.09t (r = 0.98), where [Leu]t is the leucine plasma level (μmol/L) at time t (h) during CECRT and [Leu]i corresponds to the initial plasma level. From h 4 to the end of CECRT, the change in leucine plasma levels was best described by a second exponential curve defined by the equation [Leu]t = [Leu]i × 0.74 × 10-0.05t (r = 0.97).
Leucine plasma kinetic modeling obtained from seven neonates with severe acute-onset MSUD treated with CECRT and with specific nutrition. The number of patients, the mean plasma leucine level decrease, and the SD (percentage of initial plasma leucine level) are provided for each value plotted. The leucine reduction rate is correlated with a bicompartmental model. The first 3-h period corresponds to an exponential curve: [Leu]t = [Leu]i × 0.95 × 10-0.09t (r = 0.98); and the period from the h 4 to the end of CECRT corresponds to a second exponential curve: [Leu]t = [Leu]i × 0.74 × 10-0.05t (r = 0.97), where [Leu]t is the leucine plasma level (μmol/L), t (h) is CECRT duration, and [Leu]i is the initial plasma level.
After May 2000, three neonates underwent four CECRT sessions for acute-onset MSUD and their leucine plasma levels were plotted on the previously established leucine kinetic modeling curve (Fig. 2). The actual leucine levels obtained from these patients were compared with the levels predicted by the above equations (Table 3) and were found to be very similar to those predicted by the model. Calculated values derived from the model were slightly higher than the measured level obtained at the 3-h time point of CECRT and the deviation ratio was below 10% at the end of CECRT.
Patient 9 required two CECRT sessions. His initial plasma leucine level obtained was likely factitiously elevated due to a severe 12% dehydration that may have contributed to the difference observed between measured and calculated leucine blood levels in Table 3. The first CECRT session was performed after rehydration and interrupted due to filter clotting after 3 h. At the time of cessation, the leucine plasma level was below the established indications for CECRT (1388 μM, see Table 2) and specific nutritional support alone was continued. Subsequently, clinical status did not improve, leucine plasma levels rose to 1680 μM within 12 h and a second CECRT session was indicated (Table 2).
Leucine distribution volume.
The decrease in the plasma leucine concentration was reflected by one exponential equation during the first 3 h of CECRT and by a different exponential equation for h 4 until CECRT cessation. The volume of distribution measured in the first seven neonates was 34 ± 3% of body weight during the first 3 h and 72 ± 7% of body weight for the subsequent time period (Table 1). These values represent the distribution volume of leucine and correspond to the known values for extracellular water space and total body water in the newborn (4).
DISCUSSION
During CECRT, plasma leucine concentrations in neonates with MSUD decrease according to a bi-exponential profile similar to the handling of nonprotein bound small molecular weight solutes such as urea (5,6). Our study is the first to define the distribution volumes of leucine in neonates and to propose leucine kinetic modeling that provides reliable prediction of the plasma leucine level changes during extracorporeal removal therapy with or without nutrition, thus precluding the need to perform repeated amino acid chromatography analysis.
Several other practical advantages result from reliable kinetic modeling of plasma leucine levels. The most significant impact of this modeling is the fact that it becomes feasible to determine the optimal duration of CECRT based on the leucine plasma level at the time of NICU admission. CECRT duration can easily be calculated with this model to target a plasma leucine level below 1000 μM, which, in our experience, substantially reduces the risk of neurologic effects (3). For example, it becomes possible to determine that a 7-h CECRT session will be required to decrease the plasma leucine level below 1000 μM (a 66% decrease) in a neonate with an initial leucine plasma concentration of 3000 μM when a Miniflow 10 hemofilter is used with a blood flow of 25 mL/min and a dialysate flow of 25 mL/min without recirculation. If these settings are not used during CECRT, it can still be ascertained that, when the leucine clearance reaches values >4 mL/min, the remaining duration of the session will be <7 h regardless of the method (hemofiltration, hemodialysis, or hemodiafiltration) or hemofilter used. In fact, because creatinine and leucine are neutral molecules without protein binding and with a similar molecular weight (113 D and 131 D, respectively) and mass transfer coefficient, creatinine clearance may be used in clinical practice to estimate leucine clearance if rapid chromatography analysis is not available (7).
Our data showed that leucine is removed from an average distribution volume of 34% of body weight during the first 3 h of CECRT and from a distribution volume of 72% during the balance of CECRT duration. Butte et al. (4) reported a mean total body water space of 73% of body weight in the newborn, a value markedly similar to the leucine distribution volume measured for the second CECRT period. The leucine distribution volume estimated by Collins et al. (8) was 46.6 ± 15.4% of body weight in 10 healthy adults and similar values were obtained in 3 adults with MSUD. This figure essentially corresponds to the normal fraction of total body water in humans, which is 50–70% of body weight (9,10). Collins et al. (8) assessed the leucine distribution volume with a [1–14C] L-leucine bolus followed by plasma collection over a short period of time (120 min). When two different compartments are involved, as suggested by our data, the slow leucine diffusion from one compartment to another may result in an underestimation of the distribution volume. This hypothesis of a bicompartmental model with different kinetics in each compartment is supported by our kinetic modeling and by the follow-up of patient 9. Indeed, we observed that the leucine level in this patient increased subsequent to the first 3-h CECRT session. Although this increase could potentially be attributed to either persistent net protein catabolism and/or to transfer of leucine from the intracellular to the extracellular compartment, the latter explanation was deemed more plausible given the fact that the patient was not infected and that nutrition was well tolerated.
In our study, we did not use isotopic methods because the neonates included were not in a steady state condition (11). Instead, we estimated the leucine distribution volume by considering the leucine mass removed and the leucine plasma level decreases that occurred during CECRT. We deemed this estimation to be accurate because both urinary leucine excretion (<5 μmol/kg/h) (12) and leucine oxidation are negligible in neonates with classical MSUD (13,14). In our study, the main factor that could have affected our estimated distribution volume is protein metabolism, because proteolysis can liberate leucine from the protein pool and protein synthesis can result in the incorporation of leucine into the protein pool (Fig. 3). Protein metabolism had a negligible effect on our estimation of leucine distribution volume for the following reasons. First, changes in plasma leucine levels occur slowly with whole-body leucine turnover and at a rate much slower than that seen during CECRT. Indeed, maximal plasma leucine variations in neonates are reported to be increases in the order of 30 μM/h with predominant proteolysis (15) and decreases in the order of 20 μM/h with protein synthesis (16), changes that are markedly smaller than the 180 μM/h decreases seen with CECRT (see Table 1). Second, during CECRT, leucine turnover from proteolysis to protein synthesis results in only a slight effect on leucine plasma levels. The seven neonates included in our study were not infected, which suggests that the increased leucine plasma levels present at admission were attributable mainly to the BCAA contained in the dietary intake before admission and to a fasting state with protein catabolism secondary to neurologic impairment. Therefore, dietary intake free of BCAA associated with initiation of CECRT results in a switch from net proteolysis to net protein synthesis. This hypothesis was supported by the fact that leucine plasma levels were increasing in all seven neonates before treatment and by the fact that the decrease in leucine plasma levels was ongoing even after cessation of CECRT (data not shown).
Assessment of the distribution volume can be very helpful in clinical practice. In moderate MSUD with an acute onset, leucine is mainly removed by prompt enteral administration of a BCAA-free formula (17), a specific nutritional regimen aimed at achieving maximal leucine incorporation into protein synthesis. In such circumstances, leucine plasma level decreases may be estimated by using urinary urea excretion, initial plasma leucine levels, and patient body weight. Using the distribution volume, free body leucine mass (Nbody) is calculated from the following equation: Nbody = [Leu] × 0.72 × body weight. Using urea urinary excretion, net protein anabolism is estimated (3). Given that leucine represents 10% of mammalian proteins, leucine mass incorporated into protein synthesis can be estimated according to the following equation: Leucine mass removal by specific nutritional regimen (mmol) = net protein anabolism (mg/24 h) * 0.1/131 (leucine molecular weight). Given that both leucine mass removal by protein anabolism and total leucine body mass can be estimated, the delay required to obtain a specific leucine level can be estimated.
Our data may also be helpful in the management of older children and adults, particularly with regard to the indication for CECRT. In these patients, total water space is proportionately smaller (50–60%) and body weight is increased, thus implying there is a greater leucine pool to remove. Moreover, leucine incorporation into protein synthesis does not increase in a manner proportional to body weight in these older children and adults (13). When severe leucine intoxication occurs in these patients, it becomes essential to establish how much more slowly the leucine pool decreases when protein synthesis becomes active. Our kinetic modeling may also help to evaluate CECRT duration by utilizing a standardization of the leucine clearance to body surface. Given that a newborn has an approximate 0.2 m2 body surface area, it can be expected that leucine plasma levels in older children will decrease at a rate similar to that shown in Figure 1 if a leucine clearance of at least 35 mL/min · 1.73 m2 (4 mL/min × 1.73/0.2) is done. In a 10-y-old child with acute-onset MSUD, Hmiel et al. (7) reported a leucine clearance of 22.7 mL/min · 1.73 m2 with plasma levels dropping from an initial value of 2585 μM to a level below 1000 μM after 24 h of CECRT (61% decrease). According to our kinetic modeling and with a leucine clearance of 35 mL/min · 1.73 m2, 6–8 h would probably have been sufficient to attain a 60% decrease. Further studies are needed to confirm this hypothesis.
Given that it is now possible to estimate the effect of nutrition and CECRT efficiency on leucine removal in MSUD, several questions remain to be debated: How fast should we remove leucine if CECRT is performed in neonates? This question is of great interest given that a recent study suggests that specific nutrition alone can be a favorable option with early diagnosis (in infants aged less than 7 d) regardless of the initial leucine plasma level (17). The second question to be debated deals with the concept of a bicompartmental system, especially in the brain. A high leucine clearance (18) may mainly remove molecules from the extracellular water space and expose newborns to a sudden osmolarity shift, which could be deleterious in the context of cerebral edema. Further studies are necessary to fully evaluate the balance between the risks associated with CECRT and the risk of neurologic sequelae due to prolonged exposure to high leucine levels.
Abbreviations
- BCAA:
-
branched chain amino acids
- CECRT:
-
continuous extracorporeal removal therapy
- MSUD:
-
maple syrup urine disease
- NICU:
-
neonatal intensive care unit
- Nleu:
-
leucine mass
- Vd:
-
distribution volume
References
Saudubray J, Ogier H, Charpentier C, Depondt E, Coude F, Munnich A, Mitchell G, Rey F, Rey J, Frézal J 1984 Neonatal management of organic acidurias. Clinical update. J Inher Metab Dis 7: 2–9
Thompson GN, Butt WW, Shann FA, Kirby DM, Henning RD, Howells DW, Osborne A 1991 Continuous venovenous hemofiltration in the management of acute decompensation in inborn errors of metabolism. J Pediatr 118: 879–884
Jouvet P, Jugie M, Rabier D, Desgres J, Hubert P, Saudubray JM, Man NK 2001 Combined nutritional support and continuous extracorporeal removal therapy in the severe acute phase of maple syrup urine disease. Intensive Care Med 27: 1798–1806
Butte NF, Hopkinson JM, Wong WW, Smith EO, Ellis KJ 2000 Body composition during the first 2 years of life: an updated reference. Pediatr Res 47: 578–585
Sharma A, Espinosa P, Bell L, Tom A, Rodd C 2000 Multicompartment urea kinetics in well-dialyzed children. Kidney Int 58: 2138–2146
Vanholder R, Burgelman M, De Smet R, Voogeleere P, Ringoir S 1996 Two-pool versus single-pool models in the determination of urea kinetic parameters. Blood Purif 14: 437–450
Hmiel S, Martin RA, Landt M, Levy FH, Grange DK 2004 Amino acid clearance during acute metabolic decompensation in maple syrup urine disease treated with continuous venovenous hemodialysis with filtration. Pediatr Crit Care Med 5: 278–281
Collins JE, Umpleby AM, Boroujerdi MA, Leonard JV, Sonksen PH 1987 Effect of insulin on leucine kinetics in maple syrup urine disease. Pediatr Res 21: 10–13
Smith D, Engel B, Diskin AM, Spanel P, Davies SJ 2002 Comparative measurements of total body water in healthy volunteers by online breath deuterium measurement and other near-subject methods. Am J Clin Nutr 76: 1295–1301
Ellis KJ, Wong WW 1998 Human hydrometry: comparison of multifrequency bioelectrical impedance with 2H2O and bromine dilution. J Appl Physiol 85: 1056–1062
Bodamer OA, Halliday D 2001 Uses of stable isotopes in clinical diagnosis and research in the paediatric population. Arch Dis Child 84: 444–448
Schadewaldt P, Hammen HW, Ott AC, Wendel U 1999 Renal clearance of branched-chain L-amino and 2-oxo acids in maple syrup urine disease. J Inher Metab Dis 22: 706–722
Schadewaldt P, Bodner-Leidecker A, Hammen HW, Wendel U 2001 Whole-body L-leucine oxidation in patients with variant form of maple syrup urine disease. Pediatr Res 49: 627–635
Thompson GN, Bresson JL, Pacy PJ, Bonnefont JP, Walter JH, Leonard JV, Saudubray JM, Halliday D 1990 Protein and leucine metabolism in maple syrup urine disease. Am J Physiol 258: E654–E660
Schadewaldt P, Wendel U 1997 Metabolism of branched-chain amino acids in maple syrup urine disease. Eur J Pediatr 156: S62–S66
Parini R, Sereni LP, Bagozzi DC, Corbetta C, Rabier R, Narcy C, Hubert P, Saudubray JM 1993 Nasogastric drip feeding as the only treatment of neonatal maple syrup urine disease. Pediatrics 92: 280–283
Morton DH, Strauss KA, Robinson DL, Puffenberger EG, Kelley RI 2002 Diagnosis and treatment of maple syrup disease: a study of 36 patients. Pediatrics 109: 999–1008
Schaefer F, Straube E, Oh J, Mehls O, Mayatepek E 1999 Dialysis in neonates with inborn errors metabolism. Nephrol Dial Transplant 14: 910–918
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was supported by “Assistance Publique—Hôpitaux de Paris” (grant number CRC 950180) and the Hospal International.
Rights and permissions
About this article
Cite this article
Jouvet, P., Hubert, P., Saudubray, J. et al. Kinetic Modeling of Plasma Leucine Levels during Continuous Venovenous Extracorporeal Removal Therapy in Neonates with Maple Syrup Urine Disease. Pediatr Res 58, 278–282 (2005). https://doi.org/10.1203/01.PDR.0000169982.06717.67
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/01.PDR.0000169982.06717.67
This article is cited by
-
Phenylalanine flux and gastric emptying are not affected by replacement of casein with whey protein in the diet of adult cats consuming frequent small meals
BMC Veterinary Research (2014)
-
Treatment of acute decompensation of maple syrup urine disease in adult patients with a new parenteral amino‐acid mixture
Journal of Inherited Metabolic Disease (2013)
-
Duration of extracorporeal therapy in acute maple syrup urine disease: a kinetic model
Pediatric Nephrology (2006)