Abstract
Heterogeneity in respiratory syncytial virus (RSV) disease severity likely is due to a combination of host and viral factors. Infection with RSV subgroup A is thought to produce more severe disease than RSV-B. Higher RSV loads correlate with greater disease severity in hospitalized infants. Whether subgroup-specific variations in disease severity result from differences in RSV load has not been studied. A total of 102 RSV-hospitalized infants <2 y of age were studied. Nasal washes were collected in a standardized manner and were cultured in <3 h in parallel with an RSV quantitative standard in a HEp-2 plaque assay. RSV-A (72%) was more frequent than RSV-B. Disease severity risk factors were similar between subgroups. RSV loads were similar between A and B subgroups (4.77 versus 4.68 log PFU/mL). Measures of disease severity were also similar between subgroups.
Similar content being viewed by others
Main
There is extreme variability in respiratory syncytial virus (RSV) disease severity evidenced in previously healthy infants. Approximately 50% of infants are infected during their first year of life (1,2), yet only 3% of infants <1 y of age are hospitalized for RSV (3). Of RSV-hospitalized infants and children, only 10% require mechanical ventilation (4). In addition, 5–10% of those who require mechanical ventilation die (5). Furthermore, more than half of RSV-hospitalized infants and a large percentage of infants who require mechanical ventilation and even die from RSV infection are previously healthy (3,5). These striking disease severity differences cannot be explained by existing neutralizing antibody titers alone (6) and are likely due to a combination of host factors and viral factors. Although this finding is not universally confirmed (7), RSV subgroup A has been shown in several studies to produce more severe disease than RSV-B (8–12). We previously demonstrated that RSV load measured from nasal wash parallels that in the lower respiratory tracts of previously healthy infected infants in the age range for primary RSV infection (13). We also previously correlated higher nasal wash RSV loads with greater disease severity in hospitalized previously healthy infants. Infants who experience RSV infection severe enough to cause respiratory failure have ∼1 log PFU/mL greater nasal RSV load than those who do not (14). Although the relationship between RSV concentrations and disease severity is controversial (15,16), higher viral loads measured near the time of admission have also been shown to correlate with longer durations of hospitalization (17,18). Whether RSV loads vary by subgroup and whether RSV subgroup-specific variations in disease severity are the result of differences in RSV load have not been studied.
METHODS
We conducted a prospective observational study of infants who were hospitalized with RSV infection during three successive winter seasons (1997–2000). Clinical data were obtained through interviews of each infant's caregiver at the time of enrollment. Patients were included in the study when they were <2 y of age and had RSV detected at any time within the preceding 48 h before enrollment. Patients were excluded when they had a diagnosed immunodeficiency, had received corticosteroids within the preceding month, had diagnosed bronchopulmonary dysplasia, or had significant congenital heart disease or positive blood cultures. Disease severity was determined after discharge by the duration of hospitalization and whether patients during their hospitalization required intensive care unit admission or mechanical ventilation. Regarding timing of symptoms, the patient's caregiver was asked individually the date of onset of each of the following specific symptoms: 1) fever, 2) runny nose, 3) cough, and 4) difficulty breathing. Of these four symptoms, the one with the earliest onset was taken to calculate the duration of symptoms before enrollment. RSV tests were ordered at the discretion of the patient's treating physician team. Identification of RSV-infected infants was by either direct fluorescent antibody (Bartels; Trinity Biotech, Wicklow, Ireland) or rapid antigen detection (Directigen; Becton Dickinson, Sparks, MD). Fewer than 5% of these RSV identifications were via Directigen. Because the number of RSV-positive patients identified daily often outnumbered the limited daily production of mature confluent monolayers, a convenience sampling of daily eligible infants was performed. Statistical analysis was performed using standard techniques, all two tailed with level of significance set at 0.05. For normally distributed data, the t test was used. The Mann Whitney U test was used for data not normally distributed. The Fisher exact test was used for categorical data. The study was conducted with the approval of the University of Tennessee Institutional Review Board and included appropriate informed consent. Trained study personnel collected nasal aspirates using a standard quantified technique. Briefly, the patients were placed in the supine position. Nonbacteriostatic saline was instilled into one naris followed by immediate insertion of an 8-French suction catheter advanced to a depth of 5 cm and with subsequent suction aspiration during removal of the catheter. The procedure was repeated twice on each naris for a total instilled saline volume of 3 mL. Cold RSV stabilization medium (3 mL; Eagle's minimum essential medium, 7.5% sucrose, 25 mM of HEPES, 1% l-glutamine, and 1% penicillin/streptomycin) was immediately aspirated through the suction catheter and mixed with the patient's secretions. Stabilized samples were transported quickly to the laboratory for processing. A HEp-2 cell plaque assay in 12-well plates with 10-fold dilutions in triplicate was used in which plates were fixed and stained at 5 d with hematoxylin and eosin. Plaque assays were performed on fresh specimen within 3 h of specimen collection. RSV quantitative standards were run in parallel with each plaque assay to ensure quantitative reliability (13). The RSV quantitative standards were frozen (−80°C) individual-use aliquots from the supernatant of RSV A Long grown in HEp-2 cells. The virus stock was stabilized in 25% sucrose before freezing. An individual aliquot was thawed and diluted 100-fold in Eagle's minimum essential medium (with 1% l-glutamine, 25 mM of HEPES, 1% penicillin/streptomycin, and 5% fetal bovine serum) before its being used as the parallel quantitative standard. The mean value of the standards was 5.12 (SD 0.092) log PFU/mL. RSV subgroup was determined in all study specimens by both genomic and antigenic techniques. A reverse transcriptase–PCR was performed as per the manufacturer's instructions (Hexaplex Prodesse Inc., Waukesha, WI) on cultured (first passage) patient RSV isolates grown in HEp-2 cells. This assay amplifies a portion of the G gene followed by amplicon detection using an enzyme hybridization assay. In addition, a direct fluorescent antibody technique using two specific MAb [MAB8581 and MAB8582 (F protein 1b and 1c), Chemicon, Temecula, CA] were applied to fluorescent slides prepared from the cultured (first passage) patient RSV isolates.
RESULTS
A total of 102 patients were enrolled and evaluated. RSV subgroup determinations by reverse transcriptase–PCR and serology yielded 100% concordant results for all 102 specimens. Characteristics of enrolled patients are listed in Table 1. Seventy-two percent of the patients were infected with RSV-A, and the remainder (28%) were infected with RSV-B. This is a similar ratio as has been found in previously published reports (8–12). There were no significant differences in risk factors for RSV disease severity (sex, age, weight, gestational age, birth weight) between patients who were infected with the two different RSV subgroups (Table 1).
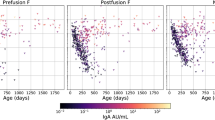
Three patients who were infected with RSV-A had received palivizumab, and two patients who were infected with RSV-B had received palivizumab. The nasal wash RSV-A and B loads measured at time of study enrollment are shown in Fig. 1A. The mean RSV-A viral load was 4.77 log PFU/mL, whereas the RSV-B viral load was 4.68 log PFU/mL. The difference between these means was 0.09 log PFU/mL. The 95% confidence intervals for RSV-B ranged from 0.7 log PFU/mL lower than RSV-A to 0.53 log PFU/mL higher than RSV-A. To evaluate whether clearance of RSV differed between subgroups, we plotted the RSV loads against the duration of symptoms (as reported by the interviewed caregiver) before specimen collection shown in Fig. 1B. When both RSV subgroups were analyzed together, greater number of days of symptoms correlated with lower initial viral load (p = 0.05). Linear regression of the individual subgroup data had lower statistical power. The slope of the individual regression line for RSV-A was −0.12 with a y-intercept of 5.27. The slope of the individual regression line for RSV-B was −0.25 with a y-intercept of 5.85 log PFU/mL. Thus, each subgroup tended to show a decline in RSV load as duration of symptoms before sample collection increased. The slopes of the individual regression lines suggest that loads of RSV-B may have declined at a faster rate, although statistically significant differences between subgroups were not achieved.
(A) Nasal wash RSV load vs subgroup in hospitalized infants. Lines and numbers on scattergrams represent means. Comparisons are by t test. The assay's maximum and minimum measurable RSV concentrations are 6.7 and 1.4 log PFU/mL, respectively. (B) RSV subgroups A and B vs duration of symptoms before collection. Data contain both RSV-A– and RSV-B–infected patients whose interview collected duration of symptom information (n = 78; RSV-A n = 58, RSV-B n = 20). The p value reflects the likelihood that the regression line includes a slope of zero. Dashed lines represent the 95% confidence intervals for possible regression lines. Because it is not compatible with known RSV disease in immunocompetent hosts, when the duration of symptoms as reported by interviewed caregiver was ≥10 d, these were considered to be erroneous reporting and therefore discarded.
Disease severity in patients who were infected with RSV-A was compared with those who were infected with RSV-B. The number of days of hospitalization for RSV-A and B, respectively was 6.6 ± 0.9 and 6.9 ± 1.3 (p > 0.05). The percentage of patients who required admission to the intensive care unit was not significantly different between subgroups (30 and 45% for RSV-A and B, respectively). Likewise, the percentage of patients who required mechanical ventilation was not significantly different between subgroups (25 and 38% for RSV-A and B, respectively).
DISCUSSION
The primary purpose of this study was to determine whether concentrations of RSV in infected infants differed with respect to infecting subgroup. The selection criteria were chosen so as to exclude patients who had conditions that would alter the relationship between disease severity and viral load. There are several limitations of this study. First, as seen in Fig. 1A, several patients had quantities of RSV that were outside the detection limitations of the plaque assay (1.4–6.7 log PFU/mL). Five RSV-A specimens were higher than the maximum measurable range, whereas only one RSV-B specimen had such a value. Thus, the specimens that were above the assay's upper detection limit predominantly cluster within the RSV-A subgroup. The upper limit of the plaque assay may have artificially reduced high viral loads in the RSV-A subgroup and thus may have reduced the ability to detect a difference in viral loads between subgroups. A quantitative virologic test with a wider dynamic range, for example, an assay based on quantitative PCR, would be helpful in evaluating this question. A second limitation concerns statistical power. Because of a relatively small number of patients with RSV-B, true differences in disease severity may not be able to be demonstrated by this study. A further limitation of this study is that only hospitalized infants were enrolled. Limiting the studied infants to those who by definition had severe RSV disease may have selected those infants with high viral loads irrespective of RSV subgroup. It is possible that differences in subgroup viral load exist with respect to propensity to induce mild disease, and this study does not address this question. In addition, limiting the patients to hospitalized infants reduces the ability to study early events in the RSV infection. We previously demonstrated that RSV loads are already declining at the time of hospitalization (13,19). Therefore, this current study does not address early subgroup-specific viral load differences. Likewise, major differences in viral load between RSV subgroups that occur in the lung cannot be excluded by this study, because the measurements of viral load were performed only in secretions from the nose. However, this is unlikely because in primary infection, viral load in the nasal wash closely parallels that recovered from the lung (deep tracheal aspirate) (13). The possibility of random error influencing the measurements and results was reduced as much as possible through the use of standardized collection techniques and designated personnel. The use of parallel RSV quantitative standards further improves assay precision and accuracy. Systematic errors are also unlikely but could be implicated in this study if subgroups had differing ability to produce plaques. RSV-B subgroup strains generally produce smaller plaques compared with strains from the A subgroup. The study was designed to control for this potential reader bias as the technicians who conducted the plaque assay were masked with respect to the identity of the RSV subgroup.
In this study, no differences in viral load between subgroups were demonstrated. Statistically it can be said with 95% confidence that within this sampled population and the time frame studied and using the described quantitative culture method, RSV loads in the upper respiratory tracts differ between subgroups by no more than 0.5–0.7 log PFU/mL. These data suggest that the previously observed severity differences between RSV subgroups are unlikely to be explained by major differences in viral load. It is possible that in a different population in which RSV subgroup was associated with a disease severity difference that significant viral load differences between subgroup might also be uncovered. Although there seems to be a relationship between higher RSV loads and greater disease severity (14,17,18), this relationship is not universally accepted (15) and needs to be defined in more detail (16). Alternatively, other viral factors may be responsible for RSV subgroup-related disease severity differences, including differences in the ability of certain viruses to promote a pathogenic inflammatory host response. In certain seasons, such factors may be linked to genomic differences of the G glycoprotein defining circulating subgroups of RSV-A and B. Indeed, genomic differences within the G glycoprotein were correlated recently with clinical disease severity (20,21), although this association has not been found universally (8). Future studies interrelating RSV sequence differences, disease severity, and viral load are needed as are studies aimed at further defining the role of viral load and viral dynamics in disease severity. In conclusion, nasal viral loads induced by natural infection of infants differ little with respect to RSV subgroup.
Abbreviations
- RSV:
-
respiratory syncytial virus
References
Glezen P, Denny FW 1973 Epidemiology of acute lower respiratory disease in children. N Engl J Med 288: 498–505
Glezen WP, Taber LH, Frank AL, Kasel JA 1986 Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child 140: 543–546
Boyce TG, Mellen BG, Mitchel EF Jr, Wright PF, Griffin MR 2000 Rates of hospitalization for respiratory syncytial virus infection among children in medicaid. J Pediatr 137: 865–870
Wang EE, Law BJ, Stephens D 1995 Pediatric Investigators Collaborative Network on Infections in Canada (PICNIC) prospective study of risk factors and outcomes in patients hospitalized with respiratory syncytial viral lower respiratory tract infection. J Pediatr 126: 212–219
Buckingham SC, Quasney MW, Bush AJ, DeVincenzo JP 2001 Respiratory syncytial virus infections in the pediatric intensive care unit: clinical characteristics and risk factors for adverse outcomes. Pediatr Crit Care Med 2: 318–323
Wang EE, Law BJ, Robinson JL, Dobson S, al Jumaah S, Stephens D, Boucher FD, McDonald J, Mitchell I, MacDonald NE 1997 PICNIC (Pediatric Investigators Collaborative Network on Infections in Canada) study of the role of age and respiratory syncytial virus neutralizing antibody on respiratory syncytial virus illness in patients with underlying heart or lung disease. Pediatrics 99( 3). Available at: www.pediatrics.org/cgi/content/full/99/3/e9
Brouard J, Freymuth F, Constantini S, Petitjean J, de Schrevel G, Duhamel JF 1993 [Prevalence and clinical aspects of A and B subgroups of respiratory syncytial virus infection. Observation of 8 consecutive epidemics between 1982 and 1990]. Arch Fr Pediatr 50: 639–643
Brandenburg AH, van Beek R, Moll HA, Osterhaus AD, Claas EC 2000 G protein variation in respiratory syncytial virus group A does not correlate with clinical severity. J Clin Microbiol 38: 3849–3852
Hall CB, Walsh EE, Schnabel KC, Long CE, McConnochie KM, Hildreth SW, Anderson LJ 1990 Occurrence of groups A and B of respiratory syncytial virus over 15 years: associated epidemiologic and clinical characteristics in hospitalized and ambulatory children. J Infect Dis 162: 1283–1290
McConnochie KM, Hall CB, Walsh EE, Roghmann KJ 1990 Variation in severity of respiratory syncytial virus infections with subtype. J Pediatr 117: 52–62
Mufson MA, Akerlind-Stopner B, Orvell C, Belshe RB, Norrby E 1991 A single-season epidemic with respiratory syncytial virus subgroup B2 during 10 epidemic years, 1978 to 1988. J Clin Microbiol 29: 162–165
Walsh EE, McConnochie KM, Long CE, Hall CB 1997 Severity of respiratory syncytial virus infection is related to virus strain. J Infect Dis 175: 814–820
Malley R, DeVincenzo J, Ramilo O, Dennehy PH, Meissner HC, Gruber WC, Sanchez PJ, Jafri H, Balsley J, Carlin D, Buckingham S, Vernacchio L, Ambrosino DM 1998 Reduction of respiratory syncytial virus (RSV) in tracheal aspirates in intubated infants using humanized monoclonal antibody to RSV F protein. J Infect Dis 178: 1555–1561
Buckingham SC, Bush AJ, DeVincenzo JP 2000 Nasal quantity of respiratory syncytial virus correlates with disease severity in hospitalized infants. Pediatr Infect Dis J 19: 113–117
Wright PF, Gruber WC, Peters M, Reed G, Zhu Y, Robinson F, Coleman-Dockery S, Graham BS 2002 Illness severity, viral shedding, and antibody responses in infants hospitalized with bronchiolitis caused by respiratory syncytial virus. J Infect Dis 185: 1011–1018
DeVincenzo JP, Buckingham SC 2002 Relationship between respiratory syncytial virus load and illness severity in children. comment J Infect Dis 186: 1376–1377; author reply 1377
DeVincenzo J, Bush A, Harrison L, Buckingham S, Murphy J, Prinz A, Young L 2001 Influence of host and viral factors in respiratory syncytial virus (RSV) duration of hospitalization. [abstract 1352]. Pediatr Res 49: 237A
DeVincenzo JP, Bush AJ, Aitken J, Harrison L 2003 Respiratory syncytial virus (RSV) load correlates with disease severity: Results of a prospective study of previously healthy naturally infected infants. Presented at the 43rd Annual Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC), Chicago, IL, September 14–17 2003
Malley R, Vernacchio L, Devincenzo J, Ramilo O, Dennehy PH, Meissner HC, Gruber WC, Jafri HS, Sanchez PJ, Macdonald K, Montana JB, Thompson CM, Ambrosino DM 2000 Enzyme-linked immunosorbent assay to assess respiratory syncytial virus concentration and correlate results with inflammatory mediators in tracheal secretions. Pediatr Infect Dis J 19: 1–7
Martinello RA, Chen MD, Weibel C, Kahn JS 2002 Correlation between respiratory syncytial virus genotype and severity of illness. J Infect Dis 186: 839–842
Fletcher JN, Smyth RL, Thomas HM, Ashby D, Hart CA 1997 Respiratory syncytial virus genotypes and disease severity among children in hospital. Arch Dis Child 77: 508–511
Acknowledgements
The author thanks Drs. Elaine Tuomanen and Malak Kotb for guidance and is grateful for the indispensable assistance of Lisa Harrison and Jody Aitken in collection of the data presented. The author also appreciates the assistance of the staff of the Le Bonheur Virology Lab and the physicians, the parents, and the infants who participated in this study.
Author information
Authors and Affiliations
Additional information
This research was funded in part by the following grants awarded to J.D.: National Institutes of Health RR16187, Children's Foundation Research Center, and the General Clinical Research Center UHPHS RR00211.
This information was presented in part as Abstract 658 at the 41st Interscience Conference on Antimicrobial Agents and Chemotherapy; Chicago, IL; December 2001.
Rights and permissions
About this article
Cite this article
Devincenzo, J. Natural Infection of Infants with Respiratory Syncytial Virus Subgroups A and B: A Study of Frequency, Disease Severity, and Viral Load. Pediatr Res 56, 914–917 (2004). https://doi.org/10.1203/01.PDR.0000145255.86117.6A
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/01.PDR.0000145255.86117.6A