Abstract
Perturbations in glucose metabolism in the fetus and in the neonate are a consistent finding in several different animal models of intrauterine growth retardation (IUGR) as well as in humans. Studies in rats who have undergone IUGR have shown decreased hepatic glycogen stores in the fetus and delayed induction of cytosolic phosphoenolpyruvate carboxykinase (PEPCK-C) at birth. Hepatic transcription factors CCAAT enhancer binding protein (C/EBP)α and C/EBPβ and the increase in cyclic AMP at birth have been implicated in the initial appearance of PEPCK-C. We have examined the effect of IUGR induced by reduced maternal inspired oxygen (fractional inspired oxygen concentration 0.14) on a) the expression of genes for hepatic C/EBPα, C/EBPβ, PEPCK-C and glycogen synthase; and b) transcription of the genes for C/EBPβ and PEPCK-C by dibutyryl cyclic AMP in the fetus. Three days (d 18-21) of decrease in maternal inspired oxygen resulted in lower maternal arterial PO2 and a lower birth weight of the pups (p < 0.01). Fetuses that underwent IUGR had significantly lower concentrations of plasma glucose, hepatic glycogen, and glycogen synthase mRNA and a higher hepatic lactate:pyruvate ratio. They also had lower levels of hepatic PEPCK-C mRNA at birth. The concentration of hepatic mRNA for C/EBPα and C/EBPβ as well as the transcription factors themselves were not affected by the decreased maternal inspired oxygen. Fetal injection of dibutyryl cyclic AMP after 24 h of decreased maternal inspired oxygen (d 18-19) had no effect on the expression of C/EBPβ. However, it resulted in an attenuated induction of PEPCK-C in the fetuses with IUGR. We speculate that a decrease in maternal inspired oxygen induced certain mediators, either in the mother or in the placenta, that caused lower fetal glucose concentration and affected the transcription of genes involved in fetal hepatic glucose metabolism. IUGR, as a result of decreased fractional inspired oxygen concentration may also be the consequence of pH-mediated changes in uterine blood flow. However, these remain to be examined in this experimental model.
Similar content being viewed by others
Main
Fetal glucose metabolism and adaptation to extrauterine life are perturbed in IUGR (1, 2). The abnormalities in fetal glucose metabolism consist of a lower blood glucose concentration and reduced hepatic glycogen content. Of significance, these observations have been made consistently in several different models of IUGR (1–4). No discernible alterations in maternal glucose kinetics could be identified in rat IUGR induced by decreased maternal inspired oxygen (4). In addition, the expression of placental glucose transporter proteins 1 and 3 also were unchanged in growth-restricted fetal rats (4, 5). Lower fetal glucose concentration has been attributed to either increased consumption of glucose by the placenta (6), or to increased utilization by fetal tissues (3). The lower glucose concentration in the immediate newborn period could also be a consequence of delayed induction of hepatic PEPCK-C activity observed in IUGR induced by bilateral uterine artery ligation (1). The mechanisms responsible for the lower hepatic glycogen levels and the delayed expression of hepatic PEPCK-C in the IUGR have not been examined.
In the fetus, hepatic glycogen accretion occurs late in gestation. Deposition of hepatic glycogen in the fetal rat begins on d 17 of gestation and reaches its highest level on d 21 (2, 7). This period of rapid glycogen accumulation coincides with a significant increase in the concentration of fetal plasma glucose and insulin, as well as an increase in the activity of glycogen synthase in the liver (8). The increase in activity of glycogen synthase is temporally related to an increase in C/EBPα mRNA, suggesting that C/EBPα may have a role in the transcription of the gene for glycogen synthase. In the present study, we have examined the relation between the expression of glycogen synthase and that of transcription factor C/EBPα.
Of special importance in the perinatal period is the initial induction of PEPCK-C gene transcription in the liver. Transcription of the gene for PEPCK-C is stimulated by cyclic AMP (cAMP)and inhibited by insulin (9). At birth, there is a decrease in the concentration of insulin and an increase in the level of glucagon and epinephrine (10, 11); the result is a rise in hepatic cAMP (9) that leads to induction of the PEPCK-C gene (12). This process is an important component in the rebound in the blood glucose concentration that occurs in all mammals in the immediate perinatal period.
The control of PEPCK-C gene transcription has been extensively studied and the putative regulatory elements in the promoter that are involved in the transcriptional response of the gene to various regulatory factors have been identified (12). A region of PEPCK-C gene promoter termed CRE2 (−148 to −137) has been implicated in the redox regulation of transcription of the PEPCK-C gene (13). However, the protein or proteins involved in binding to this region of the promoter have not been characterized. Of the major transcription factors studied to date, C/EBPα and C/EBPβ have been shown to play an important role in the cAMP and glucocorticoid regulation of PEPCK-C gene transcription (14, 15). It is presently unknown whether the transcription of the genes for C/EBPα and C/EBPβ are altered in the IUGR fetus. Additionally, the effect of IUGR on the induction of C/EBPβ and PEPCK-C by cAMP has not been examined. Because hypoxia leads to an increased concentration of the hypoxia-inducible protein, nuclear factor κB (NF-κB), and because NF-κB is also involved in the redox regulation of gene transcription, we have also quantified NF-κB in the fetal liver.
In the present study, we have examined the effect of IUGR on the expression of genes involved in the regulation of glucose homeostasis. IUGR was induced by reducing the FiO2 to 0.14 during the period of rapid fetal growth in the rat. We hypothesized that a decrease in maternal oxygen will result in lower fetal hepatic redox, which, in turn, will impair the expression of genes involved in hepatic glucose metabolism. We show that, even though it had no effect on fetal oxygen content, reduced maternal FiO2 resulted in increased hepatic lactate:pyruvate ratio, decreased expression of hepatic glycogen synthase, and attenuated induction of PEPCK-C in response to Bt2 cAMP. Additionally, it had no effect on the expression of the genes for C/EBPα and C/EBPβ.
MATERIALS AND METHODS
NAD+, NADH, ATP, ADP, AMP, lactate, pyruvate, and lactate dehydrogenase were purchased from Sigma Chemical (St. Louis, MO, U.S.A.). Bt2 cAMP was purchased from Roche Molecular Biochemicals (Summerville, NJ, U.S.A.). Quick Prep Total RNA kit was obtained from Amersham Pharmacia Biotech (Uppsala, Sweden) and [α-32P] deoxycytidine-5′-triphosphate (dCTP) and Gene Screen Plus were purchased from PerkinElmer Life Science Products (Boston, MA, U.S.A.). The C/EBPα, C/EBPβ, actin, and NF-κB antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.). The antibody to PEPCK-C was kindly provided by Dr. F.J. Ballard, GroPep Ltd. (Adelaide, Australia).
Animal Studies
Time-mated pregnant Sprague Dawley rats were purchased from Zivic-Miller (Zelienpole, PA, U.S.A.). The animals were housed in individual cages in a controlled environment, with a uniform 12-h day and night cycle. The rats had free access to standard rat chow diet and water. The animal study protocol was approved by the Institutional Animal Care Use Committee of Case Western Reserve University.
Pregnant rats (n = 4) were placed in a hypoxia chamber between d 18 and 21 of gestation, with an environmental oxygen concentration maintained at 14% (HYP) by blending nitrogen and air. The concentration of oxygen and carbon dioxide in the chamber was monitored continuously using a medical gas analyzer. The control animals (n = 4), in RA, were also placed in a similar chamber, maintained at the same rate of airflow into the chamber. Food and water were provided to the rats during the study. The food was removed from all the animals at 1700 h, the day before delivery. The animals had free access to water. Pups were delivered by cesarean section on d 21 of gestation (normal rat gestation: 22 d) after an intraperitoneal injection of ketamine (30 mg/kg) either in room air or in 14% oxygen environment. The pups in both groups were killed by decapitation at birth. The liver was freezeclamped using Wallenberger tongs and stored at −70°C. Liver samples were analyzed for redox state, adenine nucleotide concentrations, and glycogen content. The mRNA for C/EBPα, C/EBPβ, glycogen synthase, and PEPCK-C was determined, as was the concentration of NF-κB, C/EBPα, C/EBPβ, and PEPCK-C.
Changes in maternal and fetal blood gases in response to a decrease in the maternal environmental oxygen concentration were examined in a group of six animals (RA, n = 3; HYP, n = 3). Pregnant rats with an indwelling carotid artery cannulation were obtained from Zivic Miller. An arterial blood sample was collected on d 18 of gestation before placing the animal in 14% oxygen or in room air. A second blood sample was obtained on the day of delivery (d 21 of gestation). Preliminary analysis of the fetal blood gas data demonstrated that the pH, PaO2, and oxygen content were lower in fetuses in the upper portion of the uterine horn, probably as a consequence of delay in delivery of these fetuses. Therefore, blood gas data of only the first three fetuses removed from the lower part of the uterine horn were analyzed. Blood samples in heparinized capillary tubes were obtained by making an incision in the axilla, while the fetus was still attached to the placenta. Blood samples from the mother and three fetuses were collected in heparinized tubes and analyzed immediately. The fetal blood samples were analyzed individually and not pooled.
Because Bt2 cAMP administration to the fetuses in utero of normal rats has been shown to induce the expression of the PEPCK-C gene, and because C/EBPβ is important in the induction of PEPCK-C after birth (14), the effect of maternal hypoxia on the cAMP induction of PEPCK-C and C/EBPβ were examined. An additional group of pregnant Sprague Dawley rats were placed in either 14% oxygen (n = 4) or RA (n = 4) from d 18 to 19 of gestation. On d 19, after intraperitoneal ketamine injection, the abdomen was opened and Bt2 cAMP (125 mg/kg) in PBS solution was injected into the peritoneal cavity of the fetus in one horn of the uterus. A similar volume of vehicle (PBS) was injected into the fetuses in the other horn of the uterus. The abdomen was closed. Four hours later, the pups were delivered via the same incision. The pups were killed by decapitation and the livers were removed and frozen in liquid nitrogen for later analysis.
Analysis
Plasma glucose and lactate concentrations were measured using YSI 2300 Stat Glucose Lactate analyzer (YSI Inc., Yellow Springs, OH, U.S.A.). Blood oxygen content was determined using a hemoximeter assuming unchanged hematocrit (Radiometer, Copenhagen, Denmark), and pH, PCO2, and PO2 were measured using a blood gas analyzer (Ciba Corning, Medfield, MA, U.S.A.).
The effect of the reduced oxygen concentration inspired by the mother on fetal hepatic redox state and adenine nucleotide concentration was measured as follows: The livers were pulverized to a fine powder in liquid nitrogen after adding 10% perchloric acid. The tissue was homogenized in a centrifuge tube for 2 min until thawing occurred. The homogenized tissue was centrifuged at 4°C for 10 min. The pH of the supernatant was adjusted between 5 and 6 with 20% (wt/vol) potassium hydroxide (KOH). The sample was allowed to remain on ice for 30 min. Lactate and pyruvate were determined by enzymatic method of Williamson et al. (16). Adenine nucleotide concentrations were determined by HPLC (17).
Hepatic glycogen.
Preweighed (50-60 mg) liver samples were mixed with 0.5 mL 30% KOH and boiled at 98°C for 2 h. The samples were cooled to room temperature and the glycogen was precipitated by adding 5 mL 95% ethyl alcohol and 10 μL 4 M lithium chloride. After refrigerating overnight, the samples were spun at 30,000 × g for 30 min. The precipitated glycogen was hydrolyzed to glucose by adding 0.5 mL of 4 N hydrochloric acid. The glucose concentration was determined using the glucose oxidase method (Beckman glucose analyzer, Beckman Coulter, Inc., Fullerton, CA, U.S.A.). The glycogen content was expressed as millimoles glucose per gram liver (18).
RNA extraction and Northern blot analysis.
Seventy-five to 100 mg of frozen liver was homogenized and treated per the manufacturer's protocol. RNA concentrations were determined by absorbance at 260 nm using a spectrophotometer. For Northern blot analysis, a fixed amount (20 μg) of total RNA was denatured in a solution containing formaldehyde and deionized formamide, and the samples were incubated at 55°C for 15 min. RNA samples were fractionated by electrophoresis on a 1% formaldehyde-agarose gel. The integrity of the RNA was determined by ethidium bromide staining of the ribosomal subunits. RNA was then transferred to a Gene Screen Plus membrane by capillary action and cross-linked using UV light. The blots were hybridized with a randomly primed 32P-labeled, 1.1-kb PEPCK-C, 2.4-kb glycogen synthase, 2.1-kb C/EBPα, 1.5-kb C/EBPβ, and ribosomal RNA probes (19–21) for 1 h at 68°C using Quick-Hyb hybridization solution (Stratagene, La Jolla, CA, U.S.A.). Hybridized blots were washed twice for 15 min at room temperature with a 2× sodium chloride, sodium citrate-trisodium salt (SSC) buffer and 0.1% SDS solution, followed by one wash at 60°C for 30 min with a 0.1× SSC buffer and 0.1% (wt/vol) SDS for a high-stringency wash. The blots were then placed in a PhosphorImager screen and exposed for 24 h. The radioactivity was measured by using the Molecular Dynamics PhosphorImager (Sunnyvale, CA, U.S.A.).
Protein isolation for Western blotting.
Preweighed liver tissue was homogenized in ice-cold homogenizing buffer consisting of 20 mM Tris, pH 7.6, 0.1 mM EDTA, 0.5 mM EGTA, 0.1% Triton-X, 250 mM sucrose, and 50μL/5 mL protease inhibitor mixture. Homogenates were centrifuged at 14,000 rpm for 30 min at 4°C to separate cellular debris, mitochondria, nuclei, and plasma membranes. The pellet (nuclear fraction) was suspended in homogenizing buffer. The supernatant containing plasma membranes, etc., was again centrifuged at 20,000 rpm for 30 min at 4°C. The resulting supernatant (the cytosolic fraction) was removed for analysis. The concentration of the protein was determined using the Bio-Rad protein assay kit (Bio-Rad, Hercules, CA, U.S.A.). Bovine serum albumin was used as the standard.
Electrophoresis and Western blotting.
The nuclear and cytosolic fractions were sonicated for 20 s, and 20 μg of protein was diluted in 2× loading buffer containing 100 mM/L Tris-HCl, pH 6.8, 20% β-mercaptoethanol, 4% SDS, 0.2% bromophenol blue, 20% glycerol, and separated by 12.5% SDS-PAGE. Proteins were electrophoretically transferred to Immobilon-P polyvinylene difluoride membranes (Bio-Rad). The membranes were incubated in blocking buffer containing 5% nonfat dried milk in 10 mM/L Tris, pH 7.4, 150 mM/L sodium chloride, and Tween-20 (TBS-T) for 1 h at room temperature. Membranes were then incubated with primary antibody diluted in blocking buffer for 1 h as follows: C/EBPα (1:250), C/EBPβ (1:250), PEPCK (1:1000), NF-κB (1:250), and actin (1:250); and washed three times for 15 min each in TBS-T. The membranes were then incubated with secondary antibody diluted in blocking buffer for 1 h as follows: antirabbit IgG peroxidase (1:5000) for C/EBPβ and anti-goat IgG peroxidase (1:5000) for the others. Membranes were washed three times for 15 min each in TBS-T. All incubations were at room temperature. Immunoreactive proteins were detected using the Super Signal Chemiluminescent Substrate kit (Pierce Chemical, Rockford, IL, U.S.A.), and the density of the immunoreactive bands was measured by scanning densitometry (GS-710, Imaging Densitometer; Bio-Rad).
Western blots were first reacted with C/EBPα, stripped as described by the manufacturer (Pierce Chemical), and reacted again with C/EBPβ and PEPCK-C. Separate blots were prepared for NF-κB. All the blots were normalized using actin.
Data analysis.
The concentrations of mRNA for glycogen synthase, C/EBPα, C/EBPβ, and PEPCK-C on the Northern blots were standardized using 18S rRNA The mRNA/rRNA ratio for each gene in the RA group was considered as 1. The data of the HYP group was expressed relative to the RA samples at birth. Samples from one litter were analyzed on one blot. A similar procedure was used for the experiments examining the effect of Bt2 cAMP on C/EBPα, C/EBPβ and PEPCK-C mRNA. The ratio of mRNA/rRNA of vehicle (PBS) group in RA was considered as 1. The data for cAMP groups (HYP and RA) were expressed relative to the controls (fetuses in RA group who received vehicle PBS). The Western blot data were also analyzed in a similar manner and normalized to actin.
The data presented are mean ± SEM. ANOVA and t test were used to compare the RA and HYP groups. A two-tailed p value of <0.05 was considered statistically significant.
RESULTS
Maternal food intake (RA: 52 ± 11, n = 4; HYP: 43 ± 14 g, n = 4) and weight gain (RA: 18 ± 12 g, n = 4; HYP: 15 ± 10 g, n = 4) during the experimental period (3 d) were similar between the RA and HYP groups. The litter size was also similar between the RA and HYP groups (RA: 11 ± 1, n = 4; HYP: 13 ± 1, n = 4). A decrease in the maternal inspired oxygen concentration during late gestation resulted in a significant decrease in fetal mass (RA: 4.7 ± 0.1 g; HYP: 4.2 ± 0.1 g; p < 0.01). The mass of the placenta was similar between the groups (RA: 0.41 ± 0.07 g; HYP: 0.44 ± 0.08 g).
Maternal and fetal blood gases.
On d 18 of gestation, the maternal arterial pH, PCO2, PO2, and oxygen content were similar in the RA and HYP groups. Prolonged reduction in the environmental oxygen concentration resulted in a significant increase in arterial blood pH (p < 0.05) and a decrease in PCO2 and PO2 in mothers in the HYP group (Table 1). The blood oxygen content in the RA and HYP groups was similar, suggesting that in the HYP group at a lower PO2, the Hb oxygen saturation was of similar magnitude as in the RA group (Table 1). Control animals placed in room air in a similar chamber with a similar rate of airflow showed no change in arterial blood gases. The blood pH was lower in fetuses of mothers exposed to hypoxia as compared with the RA group (p < 0.05).
Metabolic changes.
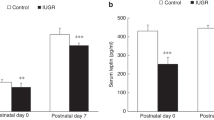
The maternal blood glucose concentration was similar in the RA and HYP groups. At delivery, the concentration of glucose in the plasma of pups in the RA group was 3.3 ± 0.5 mmol/L. Exposure of the mother to 14% oxygen resulted in a significant (p < 0.05) decrease in fetal plasma glucose concentration (1.6 ± 0.1). There was no significant difference in the fetal blood lactate concentration in the two groups (RA: 11.5 ± 0.8 mmol/L; HYP: 12.5 ± 0.8 mmol/L). The level of glycogen in the fetal liver was significantly (p < 0.05) lower in the HYP group (Fig. 1).
Fetal plasma glucose, hepatic glycogen content, and glycogen synthase mRNA in RA (open bars) and HYP (filled bars) animals. Pregnant rats were placed either in room air (RA, n = 4) or in FiO2 0.14 (HYP, n = 4) between d 18 and 21 of gestation, and pups were delivered on d 21. Blood samples and liver were obtained from the fetuses (three to four pups from each litter). Twenty micrograms of RNA was loaded in each lane and the blots were hybridized with glycogen synthase cDNA probe. The data are expressed as mean ± SEM. *Significantly different from RA group (p < 0.05).
Hepatic redox state and the concentration of adenine nucleotide in the fetal liver.
The lactate concentrations in the livers of pups in the HYP group were significantly higher (p < 0.005) when compared with the RA animals, whereas the pyruvate concentrations were not different between the groups (Table 2). The hepatic lactate:pyruvate ratio was significantly higher (p < 0.03) in the HYP group. There was no significant difference in fetal hepatic concentration of ATP, ADP, AMP, and the adenylate charge between the groups.
Hepatic glycogen synthase and PEPCK-C.
The levels of mRNA for glycogen synthase were significantly lower (p < 0.05) in the livers of fetuses in the HYP group (Fig. 1). As anticipated, birth was associated with an induction of the gene for PEPCK-C in the livers of the RA group. The level of PEPCK-C mRNA was significantly lower (p < 0.05) in the HYP group as compared with controls (Fig. 2). The concentration of the protein for hepatic PEPCK-C (61 κDa) was similar in the HYP and RA controls (Fig. 3).
Effect of reduced maternal inspired oxygen on PEPCK-C, C/EBPα. and C/EBPβ mRNA in fetal liver. Pregnant rats were placed either in room air (RA, open bars, n = 4) or in FiO2 0.14 (HYP, filled bars, n = 4) between d 18 and 21 of pregnancy, and the pups were delivered on d 21. Fetal livers were removed at birth and frozen in liquid nitrogen. RNA was extracted and 20 μg was loaded in each lane; the blots were hybridized with C/EBPα, C/EBPβ, and PEPCK-C cDNA probes. The data are expressed as mRNA levels relative to RA at birth, mean ± SEM from four litters (three to four pups each). *Significantly decreased compared with RA group (p < 0.05).
Effect of maternal hypoxia on C/EBPα, C/EBPβ, and PEPCK-C in the fetal livers. The experimental design was the same as in Figure 2. The concentration of C/EBPα and C/EBPβ in the nuclear fractions and PEPCK-C in the cytoplasmic fraction from the fetal liver was determined by Western blotting using specific antibodies. Representative Western blots are shown in the upper panel. The values presented are the densitometric units normalized for actin in each sample. The data presented is mean ± SEM from four litters (three to four pups each). RA, open bars; HYP, filled bars.
Hepatic C/EBPα, C/EBPβ, and NF-κB.
At birth, the mRNA levels for C/EBPα and C/EBPβ in the livers of HYP pups were similar to the RA controls (Fig. 2). The concentration of proteins for hepatic C/EBPα (47 kD) and C/EBPβ (42 kD) in pups born to mothers exposed to prolonged hypoxia was also comparable to the RA animals (Fig. 3). There was no effect of decreased maternal inspired oxygen on the levels of NF-κB protein in the fetal liver.
Effect of Bt2 cAMP on the levels of mRNA and protein for hepatic C/EBPα, C/EBPβ and PEPCK-C.
Twenty-four hours after maternal exposure to low inspired oxygen, injection of Bt2 cAMP to the fetuses on d 19 of gestation did not effect the levels of C/EBPα mRNA. It also had no impact on the concentration of C/EBPα protein (data not shown). The levels of C/EBPβ mRNA (Fig. 4) and protein (Fig. 5) were also unchanged in response to Bt2 cAMP. The fetuses in the RA group demonstrated a 2.6-fold increase in the levels of PEPCK-C mRNA in response to Bt2 cAMP. Reduced inspired oxygen concentration to the mother suppressed the expression of the PEPCK-C gene in response to Bt2 cAMP in the fetal liver (Fig. 4). Injection of Bt2 cAMP did not effect the concentration of PEPCK-C protein in either group.
Effect of maternal hypoxia on Bt2 cAMP induction of PEPCK-C mRNA. Pregnant rats were placed in either room air (RA, n = 4) or in Fio2 0.14 (HYP, n = 4) between from d 18 to 19 of pregnancy. On d 19, 125 mg/kg of Bt2 cAMP (solid bars) was injected into the fetal rats in one horn of the uterus. Fetuses in the opposite horn were injected with PBS (open bars) alone. The pups were delivered 4 h later; the liver was removed and frozen. Twenty micrograms of RNA was loaded per lane and hybridized with C/EBPβ and PEPCK-C cDNA probes. The data are expressed as the mean ± SEM from four litters (three pups each). *Significantly less compared with the RA group (p < 0.05).
Effect of Bt2 cAMP on C/EBPβ and PEPCK-C in the fetal liver. The experimental design was the same as in Figure 4. A representative Western blot is shown in the upper panel. Bt2 cAMP (filled bars) had no effect on the concentration of C/EBPβ and PEPCK-C proteins. PBS, open bars.
DISCUSSION
Intrauterine growth restriction induced by decreasing the inspired oxygen concentration to the mother during late gestation was associated with profound effects on glucose homeostasis and hepatic glucose metabolism in the fetus. As shown, the plasma glucose concentration, hepatic glycogen content, and the transcription of the gene for glycogen synthase was lower in the HYP group, whereas hepatic lactate:pyruvate ratio was increased. In addition, PEPCK-C mRNA was lower at birth and there was an attenuated response of the gene for PEPCK-C in the liver to Bt2 cAMP administered in utero. These changes were associated with unchanged levels of mRNA and protein for transcription factors C/EBPα and C/EBPβ.
Model.
Metabolic changes in IUGR have been examined using several different animal models. Bilateral uterine artery ligation, a mild reduction in maternal inspired oxygen concentration (FiO2 0.17) and chronic maternal hypoxia (FiO2 0.10 for 5 d) and maternal calorie deprivation have consistently resulted in IUGR (1–4), although the putative mechanism may be quite different. A milder decrease in maternal inspired oxygen concentration did not result in any demonstrable changes in glucose metabolism of the mother (4). However, it resulted in an increase in maternal-fetal glucose gradient. On the other hand, a greater magnitude of reduction in maternal inspired oxygen concentration (FiO2 0.10 for 5 d) led to maternal hypoxia, hyperglycemia, and hyperinsulinemia (3). Maternal and fetal metabolic acidosis were also evident in this IUGR model. We chose an intermediate level of maternal inspired oxygen concentration (FiO2 0.14) so that the effects of reduced inspired oxygen concentration could be evident in the fetal hepatic glucose metabolism without causing major changes in maternal and fetal blood oxygen and acid-base balance. We observed that lowering the maternal inspired oxygen concentration to 14% decreased the maternal PO2 but did not alter the blood oxygen content. Similarly, it had no effect on fetal PaO2 and O2 content, although the blood pH was significantly lower in the HYP group. It should be underscored that the fetal PO2 and O2 content were measured in a mixed (arteriovenous) sample. Whether the fetal umbilical venous PO2 was also unchanged cannot be discerned from the present data. The cytosolic redox state of the fetal liver in the HYP group was lower (increased lactate:pyruvate ratio), even though the fetal O2 content was unchanged. The reduction in fetal redox suggests that decreasing maternal inspired oxygen concentration (FiO2 0.14) may have resulted in tissue hypoxia, although we could not demonstrate any change in fetal blood O2 content.
Fetal glucose concentration.
The fetal plasma glucose concentration in the present study and in other models of IUGR has been observed to be lower than controls (22–25). The mechanism of the decrease in fetal glucose concentration has not been delineated. The primary source of glucose for the fetus is that transported across the placenta from the mother. Saker et al. (4) showed no change in maternal plasma glucose concentration, no change in rates of glucose turnover in the mother, and unchanged expression of the genes for glucose transporters (GLUT1 and GLUT3) in the placenta of IUGR rats. The authors suggested that the lower concentration of glucose in the blood of fetal rats was not the consequence of decreased maternal glucose supply or of a lowered level of expression of transporters in the placenta. The decrease in fetal plasma glucose cannot be related to changes in plasma insulin levels. Studies in animal models and in humans have consistently demonstrated that the insulin levels are lower in the IUGR fetus in the presence of lower plasma glucose concentration (22–25). Because of the small blood samples obtained, we could not measure insulin levels in our study. However, because fetal glucose concentration was lower, we would have anticipated corresponding lower fetal insulin levels. The only exceptions are the data of Lueder et al. (3), who observed normal fetal glucose concentration and high insulin levels. In their study, high insulin levels were also associated with an increased utilization of glucose by selected fetal tissues (lung, heart, and kidney). Whether a reduction in maternal inspired oxygen concentration to 14% used in the present study also led to increased glucose consumption by the placenta or other tissues, and consequently resulted in fetal hypoglycemia, remains to be examined.
Regulation of fetal hepatic glycogen metabolism.
Previous studies have shown that the concentrations of plasma glucose and hepatic glycogen are lower in newborn animals with IUGR when compared with controls (2, 26). However, the mechanisms responsible for these changes have not been identified. The fetal liver in the rat and other mammalian species exclusively utilizes glucose as a substrate for glycogen synthesis. Alterations in the concentration of blood glucose in the fetus could affect the accretion of hepatic glycogen. Gruppuso and Brautigan (2) showed a decreased glycogen content in the presence of a lower fetal plasma glucose concentration in an IUGR model, whereas higher hepatic glycogen stores were associated with elevated plasma glucose concentration in fetuses of mothers with streptozotocin-induced diabetes (27). These data suggest that the concentration of glucose in the blood of fetal rats, either directly or via modulating glycogen synthase activity, influences the accretion of glycogen in the fetal liver. In the present study, lower hepatic glycogen was associated with lower plasma glucose concentration and lower mRNA for glycogen synthase. The activity of glycogen synthase has been shown to be modulated by glucose, glucose-6-phosphate, and insulin. In adult animals, higher levels of plasma insulin in the presence of normal glucose concentration increase the activity of glycogen synthase in the liver (8). In contrast, high levels of both plasma glucose and insulin are required to enhance the activity of glycogen synthase and increase glycogen stores in the fetal liver (28). Because the concentration of insulin in the blood is determined by the prevailing glucose levels, it is possible that the observed decrease in plasma glucose concentration in the IUGR fetus in our study resulted in lower insulin levels, which, in turn, lowered the transcription of the gene for glycogen synthase. The transcription factor C/EBPα has been suggested to modulate the expression of the gene for glycogen synthase (29). The glycogen synthase mRNA has been shown to be lower at birth in the C/EBPα knock-out mouse (30). In the present study, in the IUGR fetuses, the hepatic C/EBPα mRNA levels were unchanged compared with controls in the presence of lower glycogen synthase mRNA, suggesting that the lower transcription of glycogen synthase occurred independent of C/EBPα.
Regulation of PEPCK-C.
Hypoglycemia in the IUGR newborn animals and humans also has been attributed to impaired gluconeogenesis as a consequence of a delay in the induction and lower activity of PEPCK-C (1, 31, 32). Birth is associated with an increase in the levels of plasma glucagon and catecholamines and a decrease in insulin levels (10, 11). These result in elevated levels of cAMP in the liver (9) and the initial expression of the gene for PEPCK-C at birth (33). Bussey et al. (1) observed that the appearance of PEPCK-C in the livers of newborn IUGR rats was delayed despite elevated levels of plasma glucagon. However, the administration of exogenous glucagon resulted in an early appearance of PEPCK-C. The lack of response of newborn IUGR rats to elevated endogenous glucagon levels may have been the result of lower steady state levels of cAMP. Studies using hepatoma cells have shown that cAMP is also required for the stabilization of the hepatic PEPCK-C mRNA (33). In the present study, we found lower levels of mRNA for PEPCK-C in the livers of newborn IUGR rats. It is possible that a decrease in the levels of hepatic cAMP could have altered both the transcription and stabilization of mRNA, resulting in a lower concentration of PEPCK-C mRNA in the HYP group. Although hepatic cAMP levels have not been measured in IUGR rats, studies by Lane et al. (34) and Pennington (35) have demonstrated decreased cAMP levels in the brain in the rat and chick models of IUGR.
Our data also show an increase in lactate:pyruvate ratio in the fetal liver in the HYP group, suggesting a decrease in cytosolic redox state. Studies in vitro have shown that PEPCK-C gene transcription is also regulated via a putative oxygen response element in the 5' flanking region of the promoter (13).
The promoter of the PEPCK-C gene contains binding sites for the hepatic-enriched transcription factors, C/EBPα and C/EBPβ (12). Several studies have shown that these transcription factors are necessary for the induction of the PEPCK-C gene in the newborn period (14, 15). In the present study, we did not find differences in the levels of mRNA and protein for C/EBPα and C/EBPβ between the RA and HYP groups, indicating that the observed decrease in the levels of PEPCK-C mRNA in the hypoxia group cannot be attributed to an altered expression of these genes.
Despite a lower level of PEPCK-C mRNA at birth, the PEPCK-C protein in the HYP group was similar to the RA controls. The discordance between PEPCK-C mRNA and protein may have been caused by decreased stability of PEPCK-C mRNA.
cAMP induction of PEPCK-C and C/EBPβ.
cAMP is an important intracellular signaling molecule involved in the regulation of glucose homeostasis in the neonatal period. It has also been shown to activate the dormant PEPCK-C gene in the fetal liver (12, 33, 36). We observed that maternal hypoxia suppressed the cAMP induced expression of the PEPCK-C gene in the fetal liver. The observed suppression of cAMP induced PEPCK-C mRNA levels in the IUGR fetus could have resulted from either a decreased rate of transcription of the PEPCK-C gene or increased rate of degradation (decreased stability) of the PEPCK-C mRNA. The later is unlikely, inasmuch as Hod and Hanson (33) have shown that Bt2 cAMP not only stimulates PEPCK-C gene transcription, it also extends the half-life of mRNA by blocking degradation. The half-life of PEPCK-C mRNA in their study was extended from 30 min to 240 min by Bt2 cAMP.
The gene for C/EBPβ is induced by various stimuli (37). The CREB-binding sites in the C/EBPβ gene promoter mediate the effects of cAMP, or hormones such as glucagon that act via cAMP on the transcription of the gene (38). Our data show that Bt2 cAMP had no effect on the levels of mRNA and protein for C/EBPβ. In contrast, Croniger et al. (14) observed that injection of Bt2 cAMP and theophylline into the fetuses increased the level of C/EBPβ mRNA in the fetal liver. Because theophylline inhibits the activity of phosphodiesterases, their data suggest that the phosphodiesterases activity is an important factor in the induction of the C/EBPβ gene. Whether the activity of the phosphodiesterases differs between the normal and IUGR fetuses remains to be determined.
Insulin is a known inhibitor of PEPCK-C. Although we did not measure plasma insulin levels in the fetus, they are unlikely to be increased inasmuch as the IUGR fetuses had lower circulating glucose levels.
CONCLUSION
In summary, the expression of the genes for glycogen synthase and cAMP-induced PEPCK-C were impaired in the IUGR fetus, even though the levels of mRNA for C/EBPα, C/EBPβ, and fetal oxygen content were normal. These data demonstrate that the altered levels of mRNA for glycogen synthase and PEPCK-C in the IUGR fetus and newborn are not mediated by fetal hypoxia or lower transcription of C/EBPα and C/EBPβ genes. The increased lactate:pyruvate ratio and decreased PEPCK-C mRNA in the IUGR newborn suggest that the cytosolic redox state mediates the transcription of the gene for PEPCK-C. Whether the observed changes in hepatic glucose metabolism and restriction in fetal growth are the consequence of other mediators, either from the maternal compartment or from the placenta, needs to be examined.
Additionally, these changes could be the result of pHinduced decrease in uteroplacental blood flow. Buss et al. (39) have shown a significant decline in cotyledonary (uteroplacental) blood flow in response to an increased pH induced by oral administration of sodium bicarbonate. In another study in pregnant rabbits, a decrease in PCO2 and increased pH as a consequence of hyperventilation also resulted in a significant decline in blood flow to the placental bed (40). Together, these data suggest a role for pH in the regulation of uteroplacental blood flow. We speculate that an increase in maternal pH in the experimental group may have evoked vasoconstriction in the placental bed and led to a decline in uteroplacental blood flow. Whether a decreased uteroplacental blood flow explains all the observed changes in the fetal hepatic glucose metabolism remains to be examined.
Abbreviations
- IUGR:
-
intrauterine growth retardation
- C/EBP:
-
CCAAT enhancer binding protein
- PEPCK-C:
-
phosphoenolpyruvate carboxykinase-cytosolic
- Bt2 cAMP:
-
dibutyryl cyclic AMP
- FiO2:
-
fractional inspired oxygen concentration
- RA:
-
room air
- HYP, FiO2:
-
0.14
REFERENCES
Bussey ME, Finley S, LaBarbera A, Ogata ES 1985 Hypoglycemia in the newborn growth-retarded rat: delayed phosphoenolpyruvate carboxykinase induction despite increased glucagon availability. Pediatr Res 19: 363–367.
Gruppuso PA, Brautigan DL 1989 Induction of hepatic glycogenesis in the fetal rat. Am J Physiol 256:E49–E54.
Lueder FL, Kim SB, Buroker CA, Bangalore SA, Ogata ES 1995 Chronic maternal hypoxia retards fetal growth and increases glucose utilization of select fetal tissues in the rat. Metabolism 44: 532–537.
Saker F, Voora DM, Mahajan SD, Kilic I, Ismail-Beigi F, Kalhan SC 1999 Effect of reduced inspired oxygen on fetal growth and maternal glucose metabolism in rat pregnancy. Metabolism 48: 738–744.
Reid GJ, Lane RH, Flozak AS, Simmons RA 1999 Placental expression of glucose transporter proteins 1 and 3 in growth-restricted fetal rats. Am J Obstet Gynecol 180: 1017–1023.
Challis DE, Pfarrer CD, Ritchie JW, Koren G, Adamson SL 2000 Glucose metabolism is elevated and vascular resistance and materno-fetal transfer is normal in perfused placental cotyledons from severely growth-restricted fetuses. Pediatr Res 47: 309–315.
Devos P, Hers HG 1974 Glycogen metabolism in the liver of the foetal rat. Biochem J 140: 331–340.
Margolis RN 1983 Regulation of hepatic glycogen metabolism in pre- and postnatal rats. Endocrinology 113: 893–902.
Girard J 1990 Metabolic adaptations to change of nutrition at birth. Biol Neonate 58: 3–15.
Ktorza A, Bihoreau MT, Nurjahan N, Picon L, Girard J 1985 Insulin and glucagon during the perinatal period: secretion and metabolic effects on the liver. Biol Neonate 48: 204–220.
Mayor F, Cuezva JM 1985 Hormonal and metabolic changes in the perinatal period. Biol Neonate 48: 185–186.
Hanson RW, Reshef L 1997 Regulation of phosphoenolpyruvate carboxykinase (GTP) gene expression. Annu Rev Biochem 66: 581–611.
Bratke J, Keitzmann T, Jungermann K 1999 Identification of an oxygen-responsive element in the 5′-flanking sequence of the rat cytosolic phosphoenolpyruvate carboxykinase-1 gene, modulating its glucagon-dependent activation. Biochem J 339: 563–569.
Croniger C, Trus M, Lysek-Stupp K, Cohen H, Liu Y, Darlington GJ, Poli V, Hanson RW, Reshef L 1997 Role of the isoforms of CCAAT/enhancer-binding protein in the initiation of phosphoenolpyruvate carboxykinase (GTP) gene transcription at birth. J Biol Chem 272: 26306–26312.
Crosson SM, Davies GF, Roseler WJ 1997 Hepatic expression of CCAAT/enhancer binding protein alpha: hormonal and metabolic regulation in rats. Diabetologia 40: 1117–1124.
Williamson DH, Lund P, Krebs HA 1967 The redox state of free nicotinamideadenine dinucleotide in the cytoplasm and mitochondria of rat liver. Biochem J 103: 514–527.
Vogt AM, Ackermann C, Noe T, Jensen D, Kubler W 1998 Simultaneous detection of high energy phosphates and metabolites of glycolysis and the Krebs cycle by HPLC. Biochem Biophys Res Commun 248: 527–532.
Lo S, Russell JC, Taylor AW 1970 Determination of glycogen in small tissue samples. J Appl Physiol 28: 234–236.
McGrane MM, Yun JS, Moorman AF, Lamers WH, Hendrick GK, Arafah BM, Park EA, Wagner TE, Hanson RW 1990 Metabolic effects of developmental, tissue-, and cell-specific expression of a chimeric phosphoenolpyruvate carboxykinase (GTP)/bovine growth hormone gene in transgenic mice. J Biol Chem 265: 22371–22379.
Timchenko NA, Harris TE, Wilde M, Bilyeu TA, Burgess-Beusse BL, Finegold MJ, Darlington GJ 1997 CCAAT/enhancer binding protein alpha regulates p21 protein and hepatocyte proliferation in newborn mice. Mol Cell Biol 27: 7353–7361.
Timchenko NA, Wilde M, Nakanishi M, Smith JR, Darlington GJ 1996 CCAAT/enhancer-binding protein alpha (C/EBP alpha) inhibits cell proliferation through the p21 (WAF-1/CIP-1/SDI-1) protein. Genes Dev 10: 804–815.
Hubinont C, Nicolini U, Fisk NM, Tannirandorn Y, Rodeck CH 1991 Endocrine pancreatic function in growth-retarded fetuses. Obstet Gynecol 77: 541–544.
Weiss PAM, Purstner P, Winter R, Lichtenegger W 1984 Insulin levels in amniotic fluid of normal and abnormal pregnancies. Obstet Gynecol 63: 371–375.
De Prins F, Van Assche A, Milner RDG 1983 C-peptide levels in amniotic fluid in experimental fetal growth retardation. Biol Neonate 43: 181–185.
De Prins FA, Van Assche FA 1982 Intrauterine growth retardation and development of endocrine pancreas in the experimental rat. Biol Neonate 41: 16–21.
Ogata ES, Paul RI, Finley SL 1987 Limited maternal fuel availability due to hyperinsulinemia retards fetal growth and development in the rat. Pediatr Res 22: 432–437.
Margolis RN, Seminara D 1988 Glycogen metabolism in late gestation in fetuses of maternal diabetic rats. Biol Neonate 54: 133–143.
Glinsmann WH, Eisen HJ, Lynch A, Chez RA 1975 Glucose regulation by isolated near term fetal monkey liver. Pediatr Res 9: 600–604.
Darlington GJ, Wang N, Hanson RW 1995 C/EBP alpha: a critical regulator of genes governing integrative metabolic processes. Curr Opin Genet Dev 5: 565–570.
Wang ND, Finegold MJ, Bradley A, Ou CN, Abdelsayed SV, Wilde MD, Taylor LR, Wilson DR, Darlington GJ 1995 Impaired energy homeostasis in C/EBP alpha knockout mice. Science 269: 1108–1112.
Haymond MW, Karl IE, Pagliana AS 1974 Increased gluconeogenic substrates in the small-for-gestational-age infant. N Engl J Med 291: 322–328.
Kalhan S, Alur P 1999 Glucose and small for gestational age infants. Indian Pediatr 36: 1205–1208.
Hod Y, Hanson RW 1988 Cyclic AMP stabilizes the mRNA for phosphoenolpyruvate carboxykinase (GTP) against degradation. J Biol Chem 263: 7747–7752.
Lane RH, Ramirez RJ, Tsirka AE, Kloesz JL, McLaughlin MK, Gruetzmacher EM, Devaskar SU 2001 Uteroplacental insufficiency lowers the threshold towards hypoxia-induced cerebral apoptosis in growth-retarded fetal rats. Brain Res 895: 186–193.
Pennington SN 1990 Molecular changes associated with ethanol-induced growth suppression in the chick embryo. Alcohol Clin Exp Res 14: 832–837.
Garcia Ruiz JP, Ingram R, Hanson RW 1978 Changes in hepatic messenger RNA for phosphoenolpyruvate carboxykinase (GTP) during development. Proc Natl Acad Sci U S A 75: 4189–4193.
Takiguchi M 1998 The C/EBP family of transcription factors in the liver and other organs. Int J Exp Pathol 79: 369–391.
Niehof M, Manns MP, Trautwein C 1997 CREB controls LAP/C/EBP beta transcription. Mol Cell Biol 17: 3600–3613.
Buss DD, Bisgard GE, Rawlings GA, Rankin JH 1975 Uteroplacental blood flow during alkalosis in the sheep. Am J Physiol 228: 1497–1500.
Leduc B 1972 The effect of hyperventilation on maternal placental blood flow in pregnant rabbits. J Physiol 225: 339–348.
Acknowledgements
The authors thank Mrs. Joyce Nolan for secretarial assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported, in part, by the following grants from the National Institutes of Health: HD11089 (S.C.K.), DK25541 (R.W.H.). P.S.P. was a trainee on the Metabolism Training Grant (DK07319).
Rights and permissions
About this article
Cite this article
Parimi, P., Croniger, C., Leahy, P. et al. Effect of Reduced Maternal Inspired Oxygen on Hepatic Glucose Metabolism in the Rat Fetus. Pediatr Res 53, 325–332 (2003). https://doi.org/10.1203/01.PDR.0000047643.26484.48
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/01.PDR.0000047643.26484.48
This article is cited by
-
Advances in Kawasaki disease
European Journal of Pediatrics (2004)