Abstract
The THORN trial was a multicenter, randomized, double-blind, placebo-controlled clinical trial to test the hypothesis that administration of triiodothyronine (T3) and hydrocortisone would decrease mortality and respiratory morbidity in preterm infants of less than 30 wk gestation. Two hundred fifty-three infants were randomized to receive either 6 μg·kg−1·d−1 of T3 with 1 mg·kg−1·d−1 of hydrocortisone or 5% dextrose (placebo) as a continuous i.v. infusion for 7 d. The dose was halved on d 5. Our first primary outcome was death or ventilator dependence at 1 wk, and the second was death or oxygen dependence at 2 wk. The overall mortality rate for both groups was 11.4%. Relative risk of death or ventilator dependence at 1 wk, treated versus placebo, was 0.87, p = 0.2, and death or oxygen dependence at 2 wk, 1.00, p = 0.9. We examined the relationship between free T3 (FT3) and free thyroxine (FT4) levels in the first 7 d and the primary outcome death or ventilator dependence at 1 wk in all 253 babies. We found significant positive correlations of p = 0.05 for FT3 and p = 0.002 for FT4. Thus the higher the FT3 and FT4 levels, the better the outcome. No beneficial effects of T3 and hydrocortisone were shown. In this study, although FT3 levels were doubled by the treatment infusion, FT4 levels were significantly suppressed. The lack of any beneficial effect of T3 in our study may be explained by suppression of FT4 in the treatment group.
Similar content being viewed by others
Main
A number of studies have demonstrated that premature babies have low levels of T3 and T4(1–5) and that hypothyroxinemia has been associated with developmental problems (6–8) and increased respiratory morbidity (9, 10). Consequently, several studies have examined thyroid hormone substitution in preterm infants, but most of these have involved small numbers of subjects. One large study of T4 given to babies of less than 30 wk gestation assessed neurodevelopmental outcome and found no overall beneficial effect (11). However mental outcome in a subgroup of T4-treated infants less than 27 wk gestation was significantly better than in the placebo infants when tested at 2 y of age. Two studies examining respiratory factors as end points, one giving T4(12) and another T3(13), have not shown any clear-cut benefit.
In spite of improvements in neonatal care during the past few decades (14), most tertiary level neonatal units have seen an increase in the incidence (15) of bronchopulmonary dysplasia (chronic lung disease) defined as an oxygen requirement at the age of 4 wk or beyond 36 wk conceptual age. Much of the increase can be attributed to the increasing survival of very premature babies born before 30 wk gestational age, which in turn raises the question of why these infants develop chronic lung disease when pulmonary surfactants are readily available. The lungs of babies born between 23 and 28 wk gestation are far from ready to undertake gas exchange for many reasons including immature structural maturity of the lung, inadequate pulmonary surfactant formation and secretion, and immature epithelial ion transport. The effects of high oxygen tensions (compared with fetal environment) and barotrauma from mechanical ventilation on the friable tissue have been implicated also (16). The pathophysiology underlying “new bronchopulmonary dysplasia” indicates an interference with septation (leading to fewer and larger alveoli) and abnormal vascular development (17). Proinflammatory cytokines and chronic chorioamnionitis have been suggested as causal factors (17).
The hypothesis underlying this study is that the lungs of very premature babies cannot make the transition sufficiently quickly from being a fetal organ that secretes liquid to the normal postnatal organ that absorbs liquid constantly. Throughout fetal life, lung liquid is secreted at 4 to 5 mL·kg−1·h−1, which at term is a volume of 300 to 500 mL passing up the trachea every day to contribute to amniotic fluid volume turnover (18, 19). Resting lung liquid volumes are maintained at approximately functional residual capacity, i.e. 30 mL/kg, and this volume of liquid and its secretion rate are important for lung growth (20). During labor, liquid secretion ceases and a rapid absorption of lung luminal liquid can occur at rates in excess of 40 mL/h, a change that is produced by the endogenous release by the fetus of epinephrine (21) and perhaps other mediators such as AVP (22), released as a result of the stress of labor. Epinephrine has been shown to activate sodium channels (ENaC) in the pulmonary epithelium, which allows the rapid removal of sodium ions and thus of water by osmotic forces (23). The functional appearance of the sodium channels during fetal development is under the synergistic control of thyroid hormone and of HC (24–26). The central role of ENaC in the transition at birth has been demonstrated in ENaC knockout mice: deletion of the gene coding for the α subunit of ENaC leads to early and universal death of homozygous “null” newborn animals, associated with absent clearance of lung liquid (27).
We propose that babies born very prematurely cannot clear their lung liquid adequately as a result of insufficient expression or activation of ENaC because their lungs have not been exposed to thyroid hormone and HC in adequate concentrations or for a sufficient period of time. In addition, it is possible that these babies may continue to secrete lung liquid after birth for, as a group, they have very low levels of thyroid hormone especially during the first few weeks of life (1–5). This in turn could lead to respiratory compromise and continued dependence on ventilatory support with its damaging effects on the lung.
We undertook a clinical study in which we infused T3 and HC into preterm infants born before 30 wk gestation to ascertain whether we could alter clinical outcome as measured by death and respiratory morbidity. We used T3, the active thyroid hormone, rather than T4 for two reasons:1) this was used in the animal studies and had been proven to be effective, and 2) postnatally most T3 is produced by peripheral monodeiodination of T4 and this process is immature in very preterm infants. We combined T3 with HC because of their synergistic effect seen in the animal studies. Cortisol blood levels may be adequate in premature babies, but there is considerable variation of blood concentrations in normal preterm (see below) and we wished to ensure that sufficient HC was present in each case.
METHODS
Four large neonatal intensive care units in London, namely Chelsea and Westminster Hospital, St. Georges' Hospital, University College Hospital, and the Whittington Hospital were involved in this trial. Ethical committee approval was obtained from each center.
Patients were recruited between January 1996 and April 1998. Our inclusion criteria were infants 1) less than 30 wk gestation—up to 29 wk and 6 d, 2) mechanically ventilated, 3) less than 5 h of age, and 4) with informed written parental consent. Any baby with a major morphologic abnormality was excluded from the trial. All babies were recruited after birth and randomized after consent was obtained.
T3 and HC—Pilot Study
A pilot study was performed to select the dose of T3 that would produce plasma levels of FT3 closer to that seen in term infants at 24 h of age (1200 pg/100 mL or 18 pmol/L) (28).
The entry criteria and the timing of the infusion (the start and duration) in the pilot study were identical to that in the main randomized trial (see below). From the animal studies we extrapolated a starting dose of 3 μg·kg−1·d−1 of T3. This, however, did not elevate plasma FT3 levels significantly. We found that 6 μg·kg−1·d−1 of T3 given as a continuous i.v. infusion produced plasma levels of FT3 > 10 pmol/L in the first 24 h. Although this was lower than that seen in term infants, it was significantly higher than that measured in our control patients. We did not increase the dose further because of the risk of inducing adverse effects, particularly suppression of FT4, which theoretically could affect neurodevelopment. In our control patients HC levels varied greatly and some babies had levels < 200 nmol/L. HC was given as a continuous infusion at 1 mg·kg−1·d−1. This produced HC levels > 400 nmol/L. Outcome was not assessed in the pilot study.
Dosage and Administration
Once the doses had been determined, liothyronine sodium (T3; Goldsheilds Pharmaceuticals Ltd., Croydon, U.K.) and hydrocortisone sodium succinate (HC; Pharmacia & Upjohn, Milton Keynes, U.K.) were added to a solution of 5% dextrose so that a rate of 0.5 mls/kg.hour of solution was equivalent to a dose of 6 μg·kg−1·d−1 T3 and 1 mg·kg−1·d−1 HC. Stabilization studies indicated that this solution was stable for up to one month. The treatment and placebo syringes were prepared by the Pharmacy Department at Charing Cross Hospital, London. The placebo was 5% dextrose alone. The “trial infusion” was started within 5 h of birth and given continuously at 0.5 mL·kg−1·h−1 for 7 d, the dose being halved to 0.25 mL·kg−1·h−1 on d 5. Although the choice of duration of treatment was arbitrary, we believed the babies should be exposed to the hormones until their own endocrine and respiratory systems had time to mature, hence 7 d. The dose was halved after 5 d for 2 d to allow a period of weaning to prevent a sudden drop in hormone levels. A continuous infusion rather than intermittent daily administration was used for two reasons:1) the half-life of T3 was found, from the pilot study, to be only a few hours, and 2) we wished to obtain a relatively constant concentration of hormones in the blood because the hormone effects in the fetal animal studies were reversible within 24 h.
Hormone Analysis
Blood was collected at less than 5 h (before start of infusion), 24, 48, and 72 h of age and again on d 7, 10, and 14. The amount taken was 1 mL from babies > 1000 g and 0.5 mL from babies < 1000 g. Plasma was separated and frozen.
As the quantity of plasma obtained from each sample was relatively small, an order of priority for hormone analysis was selected. We thought that FT3 and FT4 were the most important as these were the active hormones. Next plasma was analyzed for TT3 in the first 126 babies recruited and TT4 in the next 127 babies. Any plasma left over was then analyzed for TSH and HC. All the hormones were assayed at the endocrine department at Charing Cross Hospital, London. Commercial kit (Amerlex-MAB* kit specific for each hormone, Ortho-Clinical Diagnostics, Amersham, UK) using a radioimmunoassay technique was used for hormone assay.
Recruitment and Data Collection
All babies were recruited by one of three researchers. Exclusively these three people collected all data. A software program called TELEFORM (29) was used to create our data collection forms and database. Hand-written information was then entered directly into the database (ACCESS) by means of a scanner. The program allowed us to check and verify the information before this was stored in the database.
Clinical Data
Babies were ventilated according to the practice at the hospital of recruitment. At one hospital this involved prophylactic use of high-frequency oscillatory ventilation. Two of the four centers used high-frequency oscillatory ventilation for both prophylaxis and rescue, with the decision being made by the attending clinician. In the fourth hospital only conventional ventilation was used. In all four centers surfactant was given prophylactically, the first dose within 4 h of birth and the second 12 h later. Three of the centers used the natural surfactant Curosurf (Chiesi Farmaceutical Spa, Italy) and one the synthetic surfactant Exosurf (Glaxo-Wellcome, Brentford, UK). The dosage used was that recommended by the manufacturers.
In all four centers, babies were extubated onto continuous positive airway pressure when thought to be ready by the attending clinician.
Primary Outcomes
Our first primary outcome was death or ventilator dependence at 7 d and the second was death or oxygen dependence at 2 wk. Our secondary outcomes included mean duration of ventilation, mean duration of oxygen supplementation, and oxygen dependency at 36 wk postconceptual age. Other outcomes that were noted included mean duration of hospitalization, rate of intraventricular hemorrhage, presence of patent ductus arteriosus, and sepsis.
Statistical Methods
Randomization.
The Perinatal Trials Unit at Oxford conducted the randomization, with stratification for center and sex. Our pharmacist held the randomization code. Syringes containing either T3 and HC or placebo were prepared and numbered according to the randomization code. This number became the trial number of the baby recruited. Babies were randomized after consent.
Power calculations suggested a sample size of 262 babies to have an 80% chance of detecting a 25% reduction in our primary outcomes.
Comparison of outcomes between the two groups.
This was obtained by the χ2 test. The relative risk of each outcome and confidence interval was also calculated.
Comparison of hormone levels between the two groups.
To use the data as efficiently as possible hormone levels taken at time 0 (before start of infusion) and d 1, 2, 3, and 7 were converted into a single index using the “area under the curve.” Measurements up to d 7 were used as this represented the period of treatment. The area under the curve was calculated using the trapezium method. There were many missing values. These were estimated from the mean of the whole sample at that time. The effect of this would be to make the two groups more similar than they should be, so any bias introduced would make the difference between treatment groups smaller rather than larger. Thus the procedure was statistically conservative. Any subjects for whom there were three or more observations missing were omitted. Areas were estimated for FT3, FT4, and HC. In each case the area under the curve had a positive skew distribution, so a logarithmic transformation was used. The difference between the means for the two groups was compared using the two-sample t test, which gave us a p value and 95% confidence interval. Differences were converted to their antilogarithms to give the ratio of treated to control. The results were adjusted for the following prognostic variables: gestational age, sex, birth weight, center, antenatal steroids, chorioamnionitis, presence of preeclampsia, and PROM. Analysis was performed using Stata 5.0, (Stata Corp, College Station, TX, U.S.A.).
FT3, FT4, and HC as predictors of outcome.
For this analysis the area under the curve data for FT3, FT4, and HC were ranked and then divided into quintiles or fifths and tabulated against three outcomes: death or ventilator dependence at 7 d, oxygen dependence at 36 wk (using χ2 test), and total number of days on oxygen (using Kruskal-Wallis test). The results were adjusted for the following prognostic variables: gestational age, sex, birth weight, center, antenatal steroids, chorioamnionitis, presence of preeclampsia, and PROM.
Interim analysis.
A data-monitoring committee comprising a statistician, endocrinologist, obstetrician, and neonatologist examined the primary outcomes after 100 babies had been recruited and did not find a reason not to continue the trial. They looked at the following outcomes: death by 7 d, death by 14 d, ventilation by 7 d, and oxygen supplementation at 14 d.
RESULTS
Patient characteristics.
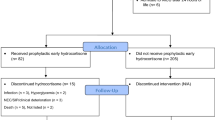
A total of 367 babies less than 30 wk gestation were identified as possible recruits. Thirty-five were excluded because mechanical ventilation was not needed. Out of the remaining 332 eligible cases, 125 babies were recruited into the treatment group and 128 into the placebo group. The two groups did not differ significantly in their baseline characteristics such as gestational age, birth weight, type of delivery, race, sex, antenatal steroids, maternal factors, PROM, type of ventilation, or surfactant given (Table 1). In 66 cases consent was refused, and in 12 cases the research team was not informed of the birth in time to recruit the infants. One infant was not recruited because of a major congenital malformation. All infants received their assigned treatment, and treatment was not discontinued in any cases.
Outcome.
Our overall mortality rate was 11%. Table 2A summarizes the relative risk of the major outcomes. There were no significant differences in the primary outcomes between the treatment and placebo groups. There was also no difference between the groups in the rate of chronic lung disease at 36 wk postconceptual age (Table 2B.
The rate of pneumothorax, pulmonary hemorrhage, intraventricular hemorrhage, periventricular leukomalacia, incidence of patent ductus arteriosus, and sepsis was similar in the two groups (Table 3). Blood pressure and heart rate were measured every 2 h for the first 48 h, and no statistical differences were noted between the treatment and placebo groups.
Mean hormone levels in the first 2 wk of life.
The changes in mean plasma hormone levels for both the treatment and placebo groups are shown in Tables 4 and 5, and a graphical representation can be found on our Web site (30). In the treatment group, FT3 levels increased from 4.9 pmol/L at less than 5 h of age to 11.5 pmol/L at 24 h and then remained at approximately this level. T3 infusion rate was halved on d 5 and stopped on d 7. FT3 levels thereafter declined and were similar to those in the placebo group by d 10 and 14.
In the treatment group, FT4 levels in the first 48 h were similar to those in the placebo group, but then declined dramatically to a nadir of 4.0 pmol/L on d 7. FT4 levels subsequently recovered to 10.7 pmol/L by d 14.
TT3 levels in the treated group increased after the start of the T3 infusion as expected and reached a peak level of 4.2 nmol/L at 48 h of age. This peak occurred a day later than that of FT3.
TT4 levels in the placebo group decreased during the first 3 d, falling from 97.2 nmol/L to 66.8 nmol/L on d 3. In the treatment group, the decrease in TT4 levels was more precipitous, falling from 91.9 nmol/L to a lowest level of 34.0 nmol/L on d 7 but recovering to 79.3 nmol/L by d 14.
TSH levels in both treatment and placebo groups were highest at birth, 8.4 and 9.2 mU/L, respectively, and then decreased to their lowest level at 48 h of age in the placebo group and 72 h of age in the treatment group. This fall was again more dramatic in the treatment group. TSH levels at d 14 were similar in both groups. Cortisol levels in the treated group were higher than in the placebo during the period of HC infusion.
Comparison of hormone levels between treatment and placebo groups for FT3, FT4, and HC levels (area under the curve) during the 7-d treatment period were compared between the two groups (statistical method described earlier). There were significant differences between the treatment and placebo groups for all three hormones. FT3 levels were twice as high (ratio of treatment to placebo, 2.1: 1; 95% confidence interval, 2.0–2.3;p < 0.0001, df = 221) and FT4 levels significantly lower (ratio of treatment to placebo, 0.79; 95% confidence interval, 0.79–0.84;p < 0.0001, df = 218) in the treatment group. Cortisol levels were also significantly higher (ratio treatment to placebo, 1.27; 95% confidence interval, 1.07–1.51;p = 0.006, df = 99) in the treatment group.
Correlation between FT3, FT4, and HC.
The correlations are presented for “area under the curve” for FT3 and FT4 in the treatment and placebo groups in Figure 1. Within each group, FT3 and FT4 are significantly positively correlated with each other, with moderate correlation coefficients (0.41, p < 0.001 for the treatment group; 0.6, p < 0.001 for the control group). For the pooled data, there is a slight negative correlation, because T3 treatment depressed FT4 (−0.13, p = 0.06). Cortisol is weakly correlated with FT3 for all patients (0.28, p = 0.003) because the treatment group received both T3 and HC and so had raised HC, but there is no evidence of such a relationship within either treatment group.
FT3, FT4, and HC as predictors of outcome.
FT3, FT4, and HC levels (area under the curve) during the 7-d treatment period were tabulated against the outcomes death or ventilator dependence at 1 wk, oxygen dependence at 36 wk postnatal age, and the median number of days in oxygen. The statistical method has been described earlier. As there was no significant difference between treatment and placebo groups, analysis of outcome was calculated for the pooled data of the two groups (that is, 253 babies). The results are presented graphically in Figure 2. The higher the FT3 and FT4, the smaller the percentage of babies dead or ventilated at 1 wk and the shorter the total number of days in oxygen. Although the proportion of babies on oxygen at 36 wk gestation fell with increasing FT3 and FT4, this was not statistically significant. Cortisol levels were associated with a U-shaped curve, i.e. both low and high cortisol levels were associated with a poorer outcome.
DISCUSSION
Direct comparison of the treatment and placebo groups for outcome did not show any significant beneficial affect of T3 and HC treatment on mortality or chronic lung disease (oxygen dependency at 36 wk). Comparison of hormone levels between the two groups showed that T3 treatment did in fact influence plasma FT3 and FT4 levels by significantly increasing FT3 levels and suppressing FT4, most likely through suppression of TSH. After cessation of T3 treatment, TSH and FT4 levels recovered and were not significantly different from those in the placebo group at d 14. We found that FT3 and FT4 levels were positively related to outcome, that is the higher the plasma FT3 or FT4 the more likely a baby was to survive or have less severe lung disease and spend less time in oxygen. This analysis took into account confounding variables such as gestational age, sex, birth weight, center, antenatal steroids, chorioamnionitis, presence of preeclampsia, and PROM. We found a bimodal association between HC and outcome, suggesting too high or too low levels are associated with an adverse outcome. It may be that the sickest babies are those with the highest HC levels and will have a bad outcome. Additionally, babies who for whatever reason (immaturity, severe illness) cannot mount a sufficient HC response will also have a poor outcome. These results cannot be explained by gestational age or birth weight as the data were adjusted for these variables.
One of the technical difficulties of this trial was obtaining all hormonal measurements on each blood sample because the amount of plasma was sometimes too small. It should be stressed that missing hormonal measurements are not preferentially from babies of lower gestational age and birth weight. For each hormone, at every time point, the mean gestational age and the mean birth weight of the babies from whom a measurement was obtained was 26 wk and 861g, respectively.
One of the entry criteria of the study was that all babies had to be on a mechanical ventilator. [This excluded a small number of babies (approximately 35) who did not require ventilation.] Our study population thus comprised the sickest individuals, but because the study was randomized at entry any beneficial effect of intervention should have been observed.
Previous trials of thyroid hormones in preterm infants have shown no significant improvement in mortality, respiratory morbidity, and neurodevelopmental outcome when either T4 or T3 have been administered alone (13, 31–33). In a large, multicenter, randomized, placebo-controlled trial of T4 in preterm infants less than 30 wk gestation, Van Wassenaer et al.(34, 35) showed that supplementation of T4 suppressed TSH and TT3 levels while elevating TT4 and FT4. This study comprised 200 infants, and T4 was given daily for 6 wk. Neurodevelopmental assessment at 2 y (8, 36) and early school age (37) showed no benefit of T4 in the whole study group, but in a subgroup of babies less than 27 wk gestation, T4 supplementation seemed to have a positive effect on neurodevelopment whereas in the subgroup of 29 wk, T4 supplementation seemed to have a negative effect. The sample sizes in the subgroups are too small to draw definite conclusions. The only trial to have shown any overall benefit is one in which both T4 and T3 were given together (38). In this trial the mortality was reduced from 29% to 6.6%, but the results must be interpreted with caution because the study population included any baby less than 37 wk gestation and only 11% of the treated group and 16% of the untreated group were < 31 wk. The majority of babies in that trial were therefore more mature and larger than the babies in our trial. Also the number of babies recruited were much smaller, 45 in the treated group, 55 in the nontreated group. Furthermore, this trial was conducted in the presurfactant era. Nevertheless, this trial suggests that both T3 and T4 may be necessary for a beneficial clinical effect. One other study that should be mentioned is that by Watterberg et al.(39), who gave continuous low-dose HC (1 mg·kg−1·d−1) for 12 d to preterm infants to prevent chronic lung disease. These investigators showed significantly higher survival without oxygen at 36 wk postconceptual age in the group of infants receiving HC. This was not found in our group of infants. The numbers of infants in the study by Watterberg et al.(39) was much smaller (40 in total) with a high rate of chorioamnionitis (70% in the treatment group and 45% in the placebo, compared with 16% in our study). The difference in the result between their study and ours is perhaps related to a smaller number of subjects or to a different pathophysiologic process.
There is considerable interest in the effects of thyroid hormones on neurodevelopmental outcome in premature babies (37, 40, 41). Our study is not yet sufficiently advanced for these data to be collected. Indications that outcome differs after thyroid hormone replacement before and after 27 wk gestation (8, 36, 37) were not known when this trial was planned and initiated. It would be statistically improper to perform post hoc subgroup analysis comparing babies before and after this gestation because our study was not designed with this in mind (42–44). However, based on work by Van Wassenaer et al.(36, 37), we investigated the possibility that treatment effect was different at different gestational ages. An interaction between treatment and gestation was sought in a logistic regression predicting ventilation or death at 7 d. The interaction was not significant (p = 0.6). This was also the case for ventilation and death at 14 d (p = 0.6) and for ventilation, oxygen, and death at 14 d (p = 0.1).
CONCLUSION
In our trial we significantly raised FT3 and TT3 levels by T3 supplementation but in doing so, suppressed FT4, TT4, and TSH levels. If high levels of both FT3 and FT4 are required for a good outcome, this may be a reason why no net beneficial treatment effect was seen in our trial. We cannot conclude from our results or previous trials that low levels of FT4 and FT3 are causally related to disease severity. Low levels of FT3 and FT4 may be just a marker of disease severity. To clarify these arguments a randomized multicenter trial needs to be undertaken supplementing both T3 and T4.
Abbreviations
- T3:
-
triiodothyronine
- T4:
-
thyroxine
- HC:
-
hydrocortisone
- FT3:
-
free T3
- FT4:
-
free T4
- PROM:
-
prolonged rupture of membranes
- TT3:
-
total T3
- TT4:
-
total T4
- ENaC:
-
epithelial sodium channel
References
Uhrmann S, Marks K, Maisels M, Friedman Z, Murray F, Kulin H, Kaplan M, Utiger R 1978 Thyroid function in the preterm infant: a longitudinal assessment. J Pediatr 92: 968–973
Mercado M, Yu VYH, Francis I, Szymonowicz W, Gold H 1988 Thyroid function in very preterm infants. Early Hum Dev 16: 131–141
Van Wassanaer AG, Kok JH, Dekker FW, Vijlder JJM 1993 Thyroid function in very preterm infants: influences of gestational age and disease. Pediatr Res 42: 604–609
Rooman RP, Du Cju MVL, Op De Beeck L, Docx P, Van Reempts P, Van Acker KJ 1996 Low thyroxinaemia occurs in the majority of very preterm newborns. Eur J Pediatr 155: 211–215
Fisher DA 1997 The hypothyroxinemia of prematurity. [editorial]. J Clin Endocrinol Metab 82: 1701–1703
Meijer WJ, Verloove-Vanhorick SP, Brand R, van den Brande JL 1992 Transient hypothyroxinaemia associated with developmental delay in very preterm infants. Arch Dis Child 67: 944–947
Reuss ML, Paneth N, Pinto-Martin JA, Lorenz JM, Susser M 1996 Transient hypothyroxinemia in preterm infants and neurodevelopmental outcome at age two years. N Engl J Med 334: 821–827
Lucas A, Rennie J, Barker BA, Morley R 1988 Low plasma triiodothyronine concentrations and outcome in preterm infants. Arch Dis Child 63: 1201–1206
Cuestas RA, Lindall A, Engel RR 1976 Low thyroid hormones and respiratory distress syndrome of the newborn. N Engl J Med 295: 297
Abbassi V, Merchant K, Abramson D 1977 Postnatal triiodothyronine concentrations in healthy preterm infants and in infants with respiratory distress syndrome. Pediatr Res 11: 802–804
Van Wassanear AG, Kok JH, Briet JM, Pijning AM, de Vijlder JJM 1999 Thyroid function in very preterm newborns: possible implications. Thyroid 9: 85–91
Amato M, Pasquier S, Carasso A, von Muralt G 1988 Postnatal thyroxine administration for idiopathic respiratory distress syndrome in preterm infants. Horm Res 29: 27–30
Amato M, Guggisberg C, Schneider H 1989 Postnatal triiodothyronine replacement and respiratory distress syndrome of the preterm infant. Horm Res 32: 213–217
Rennie JM, Roberton NRC 1999 Textbook of Neonatology. Churchill Livingstone, Cambridge, 13
Parker RA, Lindstrom DP, Cotton RB 1992 Improved survival accounts for most but not all the increase in bronchopulmonary dysplasia. Pediatrics 90: 663–668
Rennie JM, Roberton NRC 1999 Textbook of Neonatology. Churchill Livingstone, Cambridge, 663–668.
Jobe AH, Bancalari E 2001 Bronchopulmonary dysplasia. Am J Respir Crit Care Med 163: 1723–1729
Olver RE, Strang LB 1974 Ion flux across the pulmonary epithelium and the secretion of lung liquid in the fetal lamb. J Physiol (Lond) 241: 327–357
Olver RE, Schneeberger EE, Walters DV 1981 Epithelial solute permeability, ion transport and tight junction morphology in the developing lung of the fetal lamb. J Physiol (Lond) 315: 395–412
Moessinger AC, Harding RD, Adamson TM, Singh M, Kin GT 1990 Role of lung liquid volume in growth and maturation of the fetal sheep lung. J Clin Invest 86: 1270–1277
Brown MJ, Olver RE, Ramsden CA, Strang LB, Walters DV 1983 Effects of adrenaline and of spontaneous labour on the secretion and absorption of lung liquid in the fetal lamb. J Physiol (Lond) 344: 137–152
Wallace MJ, Hooper SB, Harding R 1990 Regulation of lung liquid secretion by arginine vasopressin in fetal sheep. Am J Physiol 258: R104–R111
Olver RE, Ramsden CA, Strang LB, Walters DV 1986 The role of amiloride blockable sodium transport in adrenaline induced lung liquid reabsorption in the fetal lamb. J Physiol (Lond) 376: 321–340
Barker PM, Walters DV, Markiewicz M, Strang LB 1991 Development of the lung liquid reabsorptive mechanism in fetal sheep: synergism of triiodothyronine and hydrocortisone. J Physiol (Lond) 433: 435–449
O'Brodovich H, Canessa C, Ueda J, Raffii B, Rossier BC, Edelson J 1993 Expression of the Na channel in the developing rat lung. Am J Physiol 265: C491–C496
Tchepichev S, Ueda J, Canessa C, Rossier BC, O'Brodovich H 1995 Lung epithelial Na channel subunits are differentially regulated during development and by steroids. Am J Physiol 269: C805–C812
Hummler E, Barker P, Gatzy J, Beerman F, Verdumo C, Schmidt A, Boucher R, Rossier BC 1996 Early death due to defective neonatal lung liquid clearance in alpha ENaC-deficient mice. Nat Genet 12: 325–328
Erenberg A, Phelps DL, Lam R, Fisher DA 1974 Total and free thyroid hormone concentrations in the neonatal period. Pediatrics 53: 211–216
Teleform, Automated Data Entry Forms. Cardiff Software Limited, Cardiff, Wales, UK
Biswas S, Buffery J, Enoch H, Bland M, Markiewicz, Walters D THORN trial mean hormone levels—graphical representation. http://www.mbland.sghms.ac.uk
Chowdhry P, Scanlon JW, Auerbach R, Abbassi V 1984 Results of controlled double-blind study of thyroid replacement in very low-birth-weight premature infants with hypothyroxinemia. Pediatrics 73: 301–305
Kok JH, Van Wassenaer AG, de Vijlder JJM 1995 Randomized placebo-controlled trial of thyroxine administration to infants < 30 weeks gestation in relation to mortality and morbidity. Pediatr Res 37: 218A( abstr)
Vanhole C, Aerssens P, Naulaers G, Casneuf A, Devlieger H, Van den Berghe, de Zeger F 1997 Thyroxine treatment of preterm newborns: clinical and endocrine effects. Pediatr Res 42: 87–92
Van Wassanaer AG, Kok JH, Endret E, Vulsma T, Vijlder JJM 1993 Thyroxine administration to infants of less than 30 weeks gestational age does not increase plasma tri-iodothyronine concentrations. Acta Endocrinol 129: 139–146
Van Wassanaer AG, Kok JH, Dekker FW, Endret E, Vijlder JJM 1998 Thyroxine administration to infants of less than 30 weeks gestational age decreases plasma tri-iodothyronine concentrations. Eur J Pediatr 139: 508–515
van Wassenaer AG, Kok JH, de Vijlder JJM, Briët JM, Smit BJ, Tamminga P, van Baar A, Dekker FW, Vulsma T 1997 Effects of thyroxine supplementation on neurologic development in infants born at less than 30 weeks gestation. N Engl J Med 336: 21–26
Briet JM, van Wassenaer AG, Dekker FW, de Vijlder JJ, van Baar A, Kok JH 2001 Neonatal thyroxine supplementation in very preterm children: developmental outcome evaluated at early school age. Pediatrics 107: 712–717
Schonberger W, Gremm W, Emmrich P, Gempp W 1981 Reduction of mortality rate in premature infants by substitution of thyroid hormones. Eur J Pediatr 135: 245–253
Watterberg KL, Gerdes JS, Gifford KL, Lin MH 1999 Prophylaxis against early adrenal insufficiency to prevent chronic lung disease in premature infants. Pediatrics 104: 1258–1263
Briet JM, Van Wassenaer AG, van Baar A, Dekker FW, Kok JH 1999 Evaluation of the effect of thyroxine supplementation on behavioural outcome in very preterm infants. Dev Med Child Neurol 41: 87–93
Lucas A, Morley R, Fewtrell MS 1996 Low triiodothyronine concentrations in preterm infants and subsequent intelligence quotient (IQ) at 8 year follow up. BMJ 312: 1132–1133
Altman DG, Matthews JNS 1996 Interaction 1: heterogeneity of effects. BMJ 313: 486
Matthews JNS, Altman DG 1996 Interaction 2: compare effect sizes not p values. BMJ 313: 808
Matthews JNS, Altman DG 1996 Interaction 3: how to examine heterogeneity. BMJ 313: 862
Acknowledgements
The authors thank the staff of the Neonatal Units that took part in this trial for their support and encouragement. We are indebted to the endocrine laboratory at Charing Cross Hospital London for undertaking our hormonal assays and are also particularly grateful to the Aseptic Unit at Charing Cross Hospital Pharmacy.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by a grant from the Wellcome Trust.
Rights and permissions
About this article
Cite this article
Biswas, S., Buffery, J., Enoch, H. et al. Pulmonary Effects of Triiodothyronine (T3) and Hydrocortisone (HC) Supplementation in Preterm Infants less than 30 Weeks Gestation: Results of the THORN Trial—Thyroid Hormone Replacement in Neonates. Pediatr Res 53, 48–56 (2003). https://doi.org/10.1203/00006450-200301000-00011
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-200301000-00011