Abstract
We examined the effects on astrocytes of ACTH, which is used to treat West syndrome. We stimulated cultured rat astrocytes with ACTH1–24, corticotropin-releasing factor, and dexamethasone, and examined changes in neurotrophic factor mRNAs by reverse transcription-PCR. Down-regulation of ciliary neurotrophic factor mRNA expression was observed by stimulation with ACTH1–24, but the expression of nerve growth factor, brain-derived neurotrophic factor, and nerotrophin-3 mRNAs was unaffected. Northern blot analysis revealed that the decrease in ciliary neurotrophic factor mRNA occurred 4 h after stimulation with more than 10 nM of ACTH1–24. Up-regulation of nerotrophin-3 mRNA expression was found after stimulation with 1 mM dexamethasone. These results suggest that ACTH1–24 administrated in West syndrome may influence the expression of neurotrophic factors in astrocytes in vivo.
Similar content being viewed by others
Main
ACTH is a 39-amino acid peptide that is derived from the precursor peptide pro-opiomelanocortin. Hypothalamic neurons contain most of the brain pro-opiomelanocortin–related peptides, but ACTH is also found in the amygdala, cerebral cortex, brainstem, and cerebellum (1). In addition to its classic function, ACTH is thought to act as a neurotransmitter, neuromodulator, and growth factor (1). ACTH has been demonstrated to have a neurotrophic effect, enhancing recovery from damage in both the peripheral nervous system and the CNS (2).
West syndrome is an intractable epileptic encephalopathy in infants. The seizures are lessened by ACTH and glucocorticoids, both major components of the brain-adrenal axis (3), but ACTH is reported to be more effective than glucocorticoids (4, 5). However, the mechanism of action of ACTH on immature damaged brain, which may lead to West syndrome, has not been thoroughly investigated. ACTH and adrenal steroids may be important regulators of trophic processes operative in synaptic plasticity (6). It has been reported that not only neurons but also astrocytes express ACTH-receptors and that ACTH induces morphologic changes in astrocytes (7). Thus, ACTH1–24 administrated in West syndrome may have some influence on astrocytes in vivo. Astrocytes express several kinds of neurotrophic factors and extracellular matrices that regulate axonal growth or regeneration after injury. In view of the therapy for West syndrome, it might be important to examine the influence of ACTH on astrocytes, especially with respect to the expression of neurotrophic factors.
METHODS
Astrocyte culture.
Astrocytes were prepared from d 20 embryonic Wistar rat brains according to the method of Nakanishi et al.(8). The tissues were dissected and dissociated with trypsin. The dissociated cells were suspended in low-glucose (1000 mg/L) Dulbecco's modified Eagles' medium (D-MEM, GIBCO BRL, Rockville, MD, U.S.A.) supplemented with 10% fetal bovine serum (Microbiological Associates, Sigma, St. Louis, MO, U.S.A.), penicillin G (Meiji Seika, Tokyo, Japan, 80 U/mL), and streptomycin (Meiji Seika, 0.2 mg/mL). After culturing in 25-cm2 flasks (Costar, Cambridge, MA, U.S.A.) for 6 d, the glial components were dislodged with 0.1% trypsin and replated. The medium was changed twice a week. Cultures were grown at 37°C in a humidified atmosphere of 7% CO2. After 14 d, the astrocytes formed a confluent monolayer and were prepared for the drug stimulation experiment. To check the cell population, cells were rinsed with PBS(−) and then fixed with 4% paraformaldehyde in PBS(−) for 15 min at room temperature. GFAP was visualized immunohistochemically by the streptavidin-biotin method using rabbit anti-GFAP antibody (Chemicon International Inc., Temecula, CA, U.S.A.) and Histofine SAB-PO Kit (Nichirei, Tokyo, Japan) according to manufacturer's protocol. Diaminobenzidine tetrahydrochloride was used as the chromogen and methyl green was used as a counterstain. More than 95% of the cultured cells were positive for GFAP (data not shown).
Drug stimulation experiment.
Twenty-four hours before treatment of the confluent astrocyte monolayer, the medium was replaced to serum-free high-glucose (4500 mg/L) D-MEM containing penicillin G, streptomycin, and 1% N-2 Supplement (100× N-2 Supplement, GIBCO BRL). CRF (Reseach Biochemicals International, Natick, MA, U.S.A.) in distilled water, ACTH1–24 (Calbiochem-Nova Biochem International, San Diego, CA, U.S.A.) in distilled water, or Dex (Wako Pure Chemical, Osaka, Japan) in ethanol was added to the cultures. Controls for CRF and ACTH1–24 were treated with distilled water, and those for Dex with ethanol (0.01%). To investigate whether the ACTH1–24-induced effect is mediated by activation of PKA or PKC, astrocytes were pretreated with protein kinase inhibitors, H-89 or H-7 (Seikagaku Corporation, Tokyo, Japan), for 1 h before ACTH1–24 stimulation.
Reverse transcription-PCR.
Total RNA was isolated from control and drug-stimulated astrocytes using Isogen (Wako) according to the manufacturer's instructions. Reagents used for RT-PCR were deoxyribonuclease I (DNase I, amplification grade, GIBCO BRL); 10× DNase I reaction buffer; 25 mM EDTA (GIBCO BRL); 25 mM MgCl2 (TaKaRa, Kusetsu, Japan); 10× PCR buffer (TaKaRa); dNTP mixture (TaKaRa); oligo (dT)12–18 primer (GIBCO BRL); 0.5 μg/μL Superscript II RNase H− reverse transcriptase (GIBCO BRL); and 0.1 M DTT (GIBCO BRL).
One microgram of RNA, 1 μL of 10× DNase I reaction buffer, 1 μLof DNase I, and diethylpyrocarbonate-treated water to 10 μL were mixed in a 0.5-mL tube, and the tube was incubated for 15 min at room temperature. DNase I activity was inactivated by addition of 1 μL of 25 mM EDTA and heating for 10 min at 70°C. Reverse transcription of RNA samples was performed in 20 μL of the reaction buffer containing 1 μL of oligo (dT)12–18 primer, 2 μL of 10× PCR buffer, 1 μL of 25 μM MgCl2, 2 μL of dNTP mixture, 2 μL of DTT, and 1 μL of Superscript II RNase H− reverse transcriptase. First-strand cDNAs were synthesized at 42°C for 50 min. mRNA-cDNA chains were denatured, and the reverse transcriptase activity was arrested by heating at 70°C for 10 min. Primer pairs for NGF, BDNF, NT-3, and CNTF (all Promega, Madison, WI, U.S.A.) were used for amplification and yielded a single band corresponding to 189-bp, 296-bp, 176-bp, and 168-bp DNA fragments, respectively (Table 1). The reaction was carried out for 35 cycles (NGF, BDNF, NT-3) or 27 cycles (CNTF) using a denaturing step at 94°C for 30 s, an annealing step at 65°C for 1 min, and an extension step at 68°C for 2 min. β-Actin mRNA detection was used to show equal amounts in the control and stimulated groups. The rat β-actin–specific upstream and downstream primers are listed in Table 1. They yielded a single band corresponding to a 542-bp DNA fragment. The reaction was carried out for 25 cycles, using a denaturing step at 94°C for 30 s, an annealing step at 63°C for 1 min, and an extension step at 68°C for 2 min.
Northern blot analysis.
Aliquots of total RNA (10 μg) were denatured with formaldehyde, fractionated on 0.9% agarose/2.1 M formaldehyde gels, and blotted onto nylon membranes (Micron Separations Inc., Westborough, MA, U.S.A.), which were fixed by UV irradiation. Equal loading of RNA samples was confirmed by ethidium bromide staining. Membranes were prehybridized for 18 h at 42°C in a solution containing 50% formamide, 5× standard saline citrate (SSC), 5× Denhardt's solution, 500 μg/mL salmon sperm DNA, 1% glycine, and 0.1% SDS, and hybridized at 42°C for 24 h with 32P-labeled cDNA probe for CNTF (0.6 kb). Plasmids containing rat CNTF-cDNA were kindly provided by Professor Shouei Furukawa (Laboratory of Molecular Biology, Gifu Pharmaceutical University). The hybridization solution consisted of 50% formamide, 5× SSC, 1.5× Denhardt, 100 μg/mL salmon sperm DNA, 0.1% SDS, and 10% dextran sulfate. The membranes were washed twice at room temperature for 5 min in 2× SSC containing 0.1% SDS, once at 65°C for 15 min in 2× SSC containing 0.1% SDS, and once at 65°C for 5 min in 1× SSC containing 0.1% SDS. The membranes were then exposed to x-ray Omat AR film (Eastman Chemical Company, Rochester, NY, U.S.A.) for 2 d.
Western blot analysis.
To determine the content of CNTF protein in astrocytes stimulated with ACTH1–24, cells were washed with PBS(−) and scraped into 50 μL of lysis buffer (5 mM Tris-HCl pH 7.4, 2 mM phenylmethanesulfonyl fluoride (PMSF), 10 μM pepstatin A, 10 μM leupeptin, 2 mM EDTA, and 0.5% NP-40). After sonication, total protein in the sonicates was determined using a BCA protein assay kit (Pierce, Rockford, IL, U.S.A.). For Western blot analysis, 50 μg of protein per lane was electrophoresed in 12% polyacrylamide gels. Recombinant CNTF was electrophoresed in a separate lane. After blotting on polyvinylidene difluoride membranes (Millipore, Bedford, MA, U.S.A.) for 45 min at 180 mA, the membranes were blocked with Tris-buffered saline containing 5% dry milk powder (Snow Brand, Sapporo, Japan) and incubated with anti-rat CNTF antibody (Pepro Tech, Inc., London, England) diluted 1:1000. Bound antibody was detected using anti-rabbit IgG horseradish peroxidase–linked F(ab′)2 fragment and ECL Western blotting detection reagents (Amersham Life Science, Buckinghamshire, England).
Statistical analysis.
X-ray films were scanned and analyzed using NIH image 1.59. For Northern blot analysis, film background was subtracted and the density of 28S ribosomal RNA was used for normalization. All data were entered into computer and analyzed using the StatView Program (Abacus Concepts, Berkeley, CA, U.S.A.). The effect of ACTH1–24 or drug stimulation was compared with control values using t test for Figures 1, 4, and 6, or multivariate analysis of variance (MANOVA) for Figures 2, 3, and 5. When statistical significance was observed, one-way ANOVA with Scheffépost hoc test was performed for each set of variables. Probability values less than or equal to 0.05 were considered significant.
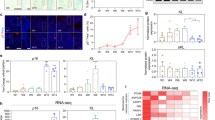
Expression of neurotrophic factor mRNAs in astrocytes treated with ACTH1–24. Cultured astrocytes from whole brain of embryonic d 20 Wistar rats were stimulated by 100 nM ACTH1–24 for 8 h. PCR products were electrophoresed on 1.5% agarose gels and visualized by ethidium bromide staining. A, PCR products from the samples with reverse transcription. B, PCR products from the samples without reverse transcription. C, values, presented as ratios relative to controls which received no ACTH1–24 treatment, are mean ± SEM (bars) of three experiments. **p < 0.01 compared with the control (t test).
Western blot analysis of intracellular CNTF protein in astrocytes treated with ACTH1–24. Astrocytes were harvested at 8 h, 24 h, and 72 h after ACTH1–24 treatment and cell extracts were subjected to Western blot analysis as described in the text. A, representative result of four experiments. B, changes in CNTF band relative to controls. Values are mean ± SEM (bars) of four experiments.
Expression of neurotrophic factors mRNAs in astrocytes treated with CRF or Dex. Cultured astrocytes were treated with 1 μM CRF (A) or 1 μM Dex (B) for 8 h. The expression of neurotrophic factor mRNAs was analyzed by RT-PCR. Pictures are representative results of three experiments. Values, presented as ratios relative to controls, are mean ± SEM (bars) of three experiments. **p < 0.01 compared with the control (t test).
Time-dependent changes in CNTF mRNA in astrocytes treated with ACTH1–24. Cultured astrocytes were exposed to 100 nM ACTH1–24 for the indicated periods of time. Total RNA was extracted and subjected to Northern blot analysis as described in the text. A, autoradiogram of a blot hybridized with CNTF probe. B, 28S ribosomal RNA in an ethidium bromide–stained gel, allowing for comparison of the total amount of RNA (10 μg) per sample. C, time-dependent changes in CNTF mRNA expression were relative to controls. Values are mean ± SEM (bars) of three experiments. **p < 0.05 compared with the control (Scheffépost hoc test).
Dose-dependent changes in CNTF mRNA in astrocytes treated with ACTH1–24. Cultured astrocytes were exposed to ACTH1–24 at indicated concentrations for 8 h. Total RNA was extracted and subjected to Northern blot analysis as described in the text. A, autoradiogram of a blot hybridized with CNTF probe. B, 28S ribosomal RNA in an ethidium bromide–stained gel. C, dose-dependent changes in CNTF mRNA expression relative to controls. Values are mean ± SEM (bars) of three experiments. **p < 0.05 compared with the control (Scheffépost hoc test).
Effect of protein kinase inhibitors on CNTF mRNA expression in astrocytes treated with ACTH1–24. Cultured astrocytes were preincubated with protein kinase inhibitors and then exposed to ACTH1–24 for 8 h. Total RNA was extracted and subjected to Northern blot analysis as described in the text. A, cells were exposed to 1 μM H-89 for 30 min before being treated with 10 nM ACTH1–24 for 8 h. B, cells were exposed to 6 μM H-7 for 30 min before being treated with 100 nM ACTH1–24 for 8 h. Pictures are representative results of three experiments. Values, presented as ratios relative to controls, are mean ± SEM (bars) of three experiments. **p < 0.05 compared with the control (Scheffé post hoc test).
RESULTS
ACTH1-24-induced expression of neurotrophic factor mRNAs in astrocytes.
NGF, BDNF, NT-3, and CNTF are well-characterized neurotrophic factors and known to be produced by astrocytes. At first, we investigated the effects of ACTH1–24 on the expression of NGF, BDNF, NT-3, and CNTF in astrocyte. We used RT-PCR to detect NGF, BDNF, NT-3, and CNTF mRNAs. Total RNA from astrocytes stimulated by 100 nM ACTH1–24 for 8 h was amplified by RT-PCR, and PCR products for NGF, BDNF, NT-3, CNTF, and β-actin were determined by electrophoresis. It had been confirmed, in advance, by RT-PCR without the transcription step, that the PCR products were not derived from concomitant DNA fragments. Figure 1 (A and B) shows the representative results of triplicate experiments. The amounts of PCR products relative to the control (n = 3) are shown in Figure 1C. There was an apparent down-regulation of CNTF mRNA caused by stimulation with 100 nM ACTH1–24.
Time-dependent reduction of CNTF mRNA caused by ACTH1-24.
To characterize the effects of ACTH1–24 on the expression of CNTF mRNA, astrocytes were incubated for 0 to 24 h with 100 nM ACTH1–24. Figure 2 shows the time-dependent changes in CNTF mRNA in response to 100 nM ACTH1–24. An apparent down-regulation of CNTF mRNA was observed after 4 h of treatment of 100 nM ACTH1–24 (Fig. 2C).
Dose-dependent reduction of CNTF mRNA caused by ACTH1-24.
Astrocytes were exposed for 8 h to various concentrations of ACTH1–24 ranging from 100 pM to 1 μM. Figure 3 shows the dose-dependent reduction of CNTF mRNA in response to treatment with ACTH1–24 for 8 h. At concentrations greater than 10 nM ACTH1–24, down-regulation of CNTF mRNA was observed (Fig. 3C).
Effect of ACTH1-24 on intracellular CNTF protein.
To investigate whether ACTH1–24 reduces intracellular CNTF protein, Western blot analyses were performed. Astrocytes were treated with 100 nM ACTH1–24 every 24 h and harvested at 8 h, 24 h, and 72 h after the first ACTH1–24 treatment, and cell extracts were subjected to Western blot analyses. Figure 4A shows a representative result of four experiments. At 72 h, after three treatments with 100 nM ACTH1–24, the band of CNTF decreased slightly compared with control, but this is not significant (Fig. 4B).
Effects of a PKA and a PKC inhibitor on down-regulation of CNTF mRNA.
ACTH1–24 has been reported to induce cAMP, which exerts its effects mostly via activation of PKA. Therefore, we determined whether the down-regulation of CNTF mRNA by ACTH1–24 treatment was mediated via PKA. Treatment of cultured astrocytes with H-89, a selective inhibitor of PKA, did not reverse the ACTH1–24-induced suppression of CNTF mRNA. Treatment of the astrocytes with H-7, an inhibitor of PKC, slightly reduced CNTF mRNA, but this is not significant (Fig. 5).
CRF- and Dex-induced expression of neurotrophic factor mRNAs in astrocytes.
To investigate the indirect effect of ACTH, we examined the effects of CRF and Dex on the expression of neurotrophic factors in astrocytes. In this study we used Dex to check the typical glucocorticoid effect on astrocyte, because Dex has approximately 8× stronger effects than prednisolone. As with ACTH1–24 treatment, RT-PCR for NGF, BDNF, NT-3, and CNTF was performed after treating with 1 μM CRF or 1 μM Dex for 8 h. An apparent up-regulation of NT-3 mRNA by treatment with 1 μM Dex was observed, but CRF had no effect on the expression of neurotrophic factor mRNAs (Fig. 6).
DISCUSSION
With our RT-PCR results we observed down-regulation of CNTF mRNA by ACTH1–24 treatment, but expression of NGF, BDNF, and NT-3 mRNAs was unaffected. Therefore, we focused on the changes in expression of CNTF mRNA induced by ACTH1–24 treatment. Northern blot analyses revealed that down-regulation of CNTF mRNA occurred in a time- and dose-dependent manner. By Western blot analysis, a slight decrease in CNTF protein was observed at 72 h. These results suggest that repeated administration of ACTH1–24, as a therapy for West syndrome, is needed for down-regulating CNTF protein because astrocytes express high amounts of CNTF, especially under reactive conditions.
The capacity to synthesize CNTF mRNA in cultures of neural tissue appears to be confined mainly to astrocytes, as cultured fibroblasts, neurons, and macrophages do not show appreciable levels of CNTF mRNA (9, 10). CNTF is a pleiotropic cytokine that supports survival or differentiation of a variety of neuronal cell types including sensory, sympathetic, and motor neurons, and it induces type-2 astrocyte differentiation in culture (11). On the other hand, CNTF has been implicated as an injury factor involved in regulating astrogliosis in the CNS (12). In a recent report, CNTF reduced survival of isolated Purkinje cells (13). Taken together with our results, administration of ACTH under the condition of gliosis after brain damage may reduce expression of CNTF to appropriate levels that may be neuroprotective.
In adult rats less than 1% of an injected dose of ACTH is found in the brain (14, 15). Recently, Brunson et al.(16) reported that administered ACTH acts directly on limbic neurons to decrease the expression of CRF. At injured lesions or after a convulsion, especially in immature brain, administered ACTH1–24 may reach astrocytes easily because of dysfunction of the blood–brain barrier. However, further research is needed to determine the permeability of immature or damaged brain to ACTH.
Melanocortin receptors have seven membrane-spanning domains coupled to adenylate cyclase (17). Five members of this receptor family have been cloned, and MC3, MC4, and MC5 receptors are expressed in brain. ACTH at concentrations from 10 nM to 1 mM can bind to these receptors and activate adenylate cyclase (18). These concentrations correspond to those that reduce CNTF mRNA in astrocytes. In this study, treatment of cultured astrocytes with H-89, a selective inhibitor of PKA, did not reverse ACTH1–24-induced suppression of the expression of CNTF mRNA. This indicates that cascades other than PKA are involved in the ACTH-induced down-regulation of CNTF mRNA and is consistent with previous reports (19, 20). Recently, Kawasaki et al.(21) reported that cAMP activates a Rap1 in a PKA-independent manner, a new cAMP-mediated signaling pathway. According to this evidence, a cAMP-Rap1 cascade might be one of the important pathways for regulation of CNTF mRNA. It has been reported that the PKC pathway is also involved in the regulation of CNTF mRNA expression (22). Therefore, we examined the effect of a PKC inhibitor on the down-regulation of CNTF mRNA caused by ACTH1–24 treatment. An inhibitor of PKC, H-7, slightly reduced CNTF mRNA, and H-7 together with ACTH1–24 showed a tendency of an additive effect on the down-regulation of CNTF mRNA. However, there is no statistical significance between control and H-7 alone, or between H-7 alone and H-7 with ACTH1–24.
It has been proposed that enhanced expression of CRF is one of the causes of West syndrome. Therefore, we investigated the effect of CRF on the expression of neurotrophic factors in astrocytes. Expression of the CRF receptor is high in extrahypothalamic regions, and astrocytes from fetal extrahypothalamus brain cell cultures contain CRF receptors that are coupled to adenylate cyclase (23). However, in this study no significant changes in NGF, BDNF, NT-3, and CNTF mRNAs were observed in astrocytes stimulated by CRF, because we used astrocytes from whole brain that might express only low levels of CRF receptors.
In addition to ACTH, glucocorticoid hormones have been used to treat West syndrome. We found that Dex induced the expression of NT-3 mRNA in cultured astrocytes. We reported previously that Dex induces lipocortin-1 mRNA in astrocytes and that lipocortin-1 has a neurotrophic effect on cultured cortical neurons (24). These data suggest that glucocorticoid induced by ACTH1–24 may also have neurotrophic effects by inducing NT-3 and lipocortin-1 in astrocytes.
CONCLUSIONS
Both ACTH and glucocorticoids have been shown to be effective in treating West syndrome, and the major effect of ACTH and glucocorticoids is down-regulation of CRF concentration in the CNS (25), but ACTH is reported to be more effective than glucocorticoids (4, 5). This study may provide evidence for one of the mechanisms by which ACTH is advantageous for treating West syndrome, as ACTH may influence the gene expression in astrocyte. In vivo studies are needed to confirm our data.
Abbreviations
- CRF:
-
corticotropin-releasing factor
- Dex:
-
dexamethasone
- RT-PCR:
-
reverse transcription-PCR
- NGF:
-
nerve growth factor
- BDNF:
-
brain-derived neurotrophic factor
- NT-3:
-
neurotrophin-3
- CNTF:
-
ciliary neurotrophic factor
- PBS(−):
-
Ca2+/Mg2+-free PBS
- GFAP:
-
glial fibrillary acidic protein
- PKA:
-
protein kinase A
- PKC:
-
protein kinase C
References
Pranzatelli MR 1994 On the molecular mechanism of adrenocorticotrophic hormone in CNS: neurotransmitters and receptor. Exp Neurol 125: 142–161
Darlington CL, Gilchrist DPD, Smith PF 1996 Melanocortins and lesion-induced plasticity in the CNS. Brain Res Rev 22: 245–257
Baram TZ, Mitchell WG, Snead OC, Horton EJ, Saito M 1992 Brain-adrenal axis hormones are altered in the CSF of infants with massive infantile spasms. Neurology 42: 1171–1175
Snead OC, Benton JW, Hosey LC, Swann JW, Spink D, Martin D, Rej R 1989 Treatment of infantile spasms with high-dose ACTH: efficacy and plasma levels of ACTH and cortisol. Neurology 39: 1027–1031
Baram TZ, Mitchell WG, Tournay A, Snead OC, Hanson RA, Horton EJ 1996 High-dose corticotropin (ACTH) versus prednisone for infantile spasms: a prospective, randomized, blinded study. Pediatrics 97: 375–379
Mocchetti I, Spiga G, Hayes VY, Isackson PJ, Colangelo A 1996 Glucocorticoids differentially increase nerve growth factor and basic fibroblast growth factor expression in the rat brain. J Neurosci 16: 2141–2148
Muriel Z, Yohar S 1992 Melanocortins stimulate proliferation and induce morphological changes on cultured rat astrocytes by distinct transducing mechanisms. Brain Res 576: 49–58
Nakanishi K, Okouchi Y, Ueki T, Asai K, Isobe I, Eksioglu Y, Kato T, Hasegawa Y, Kuroda Y 1994 Astrocytic contribution to functioning synapse formation estimated by spontaneous neuronal intracellular Ca2+ oscillations. Brain Res 659: 169–178
Rudge JS, Morrissey D, Lindsay RM, Pasnikowski EM 1994 Regulation of ciliary neurotrophic factor in cultured rat hippocampal astrocytes. Eur J Neurosci 6: 218–229
Sendtner M, Carroll P, Holtmann B, Hughes RA, Thoenen H 1994 Ciliary neurotrophic factor. J Neurobiol 25: 1436–1453
Hughes SM, Lillien LE, Raff MC, Rohrer H, Sendtner M 1988 Ciliary neurotrophic factor induces type-2 astrocyte differentiation in culture. Nature 335: 70–73
Kahn MA, Huang CJ, Caruso A, Barresi R, Nazarian R, Condorelli DF, de Vellis J 1997 Ciliary neurotropic factor activates JAK/Stat signal transduction cascade and induces transcriptional expression of glial fibrillary acid protein in glial cells. J Neurochem 68: 1413–1423
Morrison ME, Mason CA 1998 Granule neuron regulation of Purkinje cell development: striking a balance between neurotrophin and glutamate signaling. J Neurosci 18: 3563–3573
Nicholson WE, Liddle RA, Puett D, Liddle GW 1978 Adrenocorticotropic hormone biotransformation, clearance, and catabolism. Endocrinology 103: 1344–1351
Mezey E, Palkovits M, de Kloet ER, Verhoef J, de Wied D 1978 Evidence for pituitary-brain transport of a behaviorally potent ACTH analog. Life Sci 22: 831–838
Brunson KL, Khan N, Eghbal-Ahmadi M, Baram TZ 2001 Corticotropin (ACTH) acts directly on amygdala neurons to down-regulate corticotropin-releasing hormone gene expression. Ann Neurol 49: 304–312
Mountjoy KG, Robbins LS, Mortrud MT, Cone RD 1992 The cloning of a family of genes that encode the melanocortin receptors. Science 257: 1248–1251
Hol EM, Gispen WH, Bar PR 1995 ACTH-related peptides: receptors and signal transduction systems involved in their neurotrophic and neuroprotective actions. Peptide 16: 979–993
Carroll P, Sendtner M, Meyer M, Thoenen H 1993 Rat ciliary neurotrophic factor (CNTF): gene structure and regulation of mRNA levels in glial cell culture. Glia 9: 176–187
Nagao H, Matsuoka I, Kurihara K 1995 Effects of adenylyl cyclase-linked neuropeptides on the expression of ciliary neurotrophic factor-mRNA in cultured astrocyte. FEBS Lett 362: 75–79
Kawasaki H, Gregory M, Mochizuki N, Toki S, Nakaya M, Matsuda M, Housman DE, Graybiel AM 1998 A family of cAMP-binding proteins that directly activate Rap1. Science 282: 2275–2279
Nagao H, Matsuoka I, Kurihara K 1996 Effects of phorbol ester on expression of CNTF-mRNA in cultured astrocytes from olfactory bulb. Brain Res 719: 23–28
Kapcala LP, Dicke JA 1992 Brain corticotropin-releasing hormone receptors on neurons and astrocytes. Brain Res 589: 143–148
Mizuno H, Asai K, Fujita K, Uemura K, Wada Y, Moriyama A, Ogawa H, Kimura S, Kato T 1998 Neurotrophic action of lipocortin 1 derived from astrocytes on cultured rat cortical neurons. Mol Brain Res 60: 28–39
Bajorek JG, Lee RJ, Lomax P 1986 Neuropeptides: anticonvulsant and convulsant mechanisms in epileptic model and in humans. Adv Neurol 44: 489–500
Acknowledgements
The authors thank Professor Shouei Furukawa for providing rat CNTF cDNA, and Dr. Satoru Kobayashi and Manami Yamamoto for their technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by a Grant-in-Aid for Scientific Research on Priority Areas (B) and a Grant-in-Aid for Scientific Research (C) from the Ministry of Education, Science, Sports and Culture, Japan, and a Health Sciences Research Grant for Research on Environmental Health from the Ministry of Health and Welfare, Japan.
Rights and permissions
About this article
Cite this article
Kokubo, M., Asai, K., Yamamoto, N. et al. ACTH1–24 Down-Regulates Expression of Ciliary Neurotrophic Factor mRNA in Cultured Rat Astrocyte. Pediatr Res 52, 950–957 (2002). https://doi.org/10.1203/00006450-200212000-00022
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-200212000-00022