Abstract
Previous studies have shown that plasma lipoproteins are a common target of free radical-induced oxidative stress in hypoxic newborn infants. In contrast to lipids, the reaction of proteins with various oxidants during hypoxia has not been extensively studied. We tested the hypothesis that tissue hypoxia results in increased production of protein oxidation in cord blood of preterm newborns. Heparinized blood samples of 39 hypoxic and 16 control preterm newborns were obtained from the umbilical vein, after cord clamping immediately after delivery. Plasma levels of total hydroperoxide (TH), advanced oxidation protein products (AOPP), hypoxanthine (Hx), xanthine (Xa), and uric acid (UA) were measured. Higher Hx, Xa, UA, TH, and AOPP levels were found in hypoxic newborn infants than in controls. Statistically significant correlations were observed between: TH and Hx (r = 0.54, p = 0.003, n = 28), AOPP and Hx (r = 0.64, p = 0.0001, n = 27), and TH and AOPP plasma levels (r = 0.50, p = 0.02, n = 21). In summary, TH, AOPP, Hx, Xa, and UA production is increased in fetal blood during hypoxia. The more severe the hypoxia, the higher the lipid and protein damage by free radicals.
Similar content being viewed by others
Main
Imbalance between antioxidant- and oxidant-generating systems results in oxidative stress at birth. This condition has been demonstrated by reliable markers of increased FR release in severely ill newborns (1–7). High peak pentane exhalation and high plasma concentration of hydroperoxide and malondialdehyde are associated with neonatal hypoxia and adverse outcome (3, 5, 6). Oxidative stress has also been proved in the red cells of hypoxic newborns by increased intraerythrocyte free iron content (8, 9), indicating that erythrocytes are involved in FR release during neonatal hypoxia. Recent studies on the effects of FR release on protein structures prove that radical-induced damage to proteins is not only a chain-terminating process, but also a source of chain reactions that can enhance tissue damage (10–13). For instance, TH represents a measure of overall oxidative stress, given that they are the intermediate oxidative products of lipid, peptides, and amino acids. Simultaneous determination of AOPP could provide information regarding another aspect of protein involvement in FR reactions, namely, oxidized plasma proteins that have lost their oxidant properties (11). Determination of TH and protein oxidative breakdown products in plasma therefore provides information on some of the fundamental mechanisms of oxidative stress involved in the development of FR disorders of newborn babies.
We test the hypothesis that fetal tissue hypoxia results in increased generation of FR and oxidation protein products in the plasma of preterm newborns.
METHODS
Patients.
Fifty-five preterm babies (for clinical data, see Table 1) admitted consecutively to the Neonatal Intensive Care Unit (NICU) at the Institute of Preventive Pediatrics and Neonatology, University of Siena, between January 1, 1997 and January 30, 1998, were enrolled in the study. All babies with congenital malformations, inborn errors of metabolism, blood group incompatibility, sepsis, diabetic mothers, multiple gestation, and those not born in the clinic were excluded. Thirty-nine of 55 preterm newborns enrolled were regarded as hypoxic if they fulfilled at least two of the following criteria: pH ≤7.20 in the umbilical vein, Apgar score ≤6 at 5 min, and FiO2 needed for resuscitation immediately after delivery ≥0.4. These criteria, less severe than those established by the American College of Obstetricians and Gynecologists to define newborns with birth asphyxia (14), were chosen, as reported in previous papers by us (8, 9), to evaluate all hypoxic babies, even those with mild hypoxia. Fifteen of 39 hypoxic babies were reanalyzed separately to verify whether stricter criteria of hypoxia (pH <7.15 in umbilical vein, Apgar score <5 at 5 min) changed our results. Sixteen babies without signs of perinatal hypoxia were used as controls. Heparinized blood samples were obtained from the umbilical vein after cord clamping, and immediately after delivery. The study was approved by the Human Ethics Committee of the Medical Faculty, University of Siena.
Procedures.
The blood was immediately centrifuged, and all analyses of Hx, Xa, UA, AOPP, and TH were carried out in plasma within 2 h of blood sampling to avoid the effects of storage. After centrifugation, the plasma and buffy coat were removed. Hx, Xa, and UA levels were evaluated by HPLC, with a Varian Vista 5500 high-performance liquid chromatograph equipped with a variable-wavelength UV detector (Varian, model 4290). A ready-to-use prepacked column (250 × 4.6 mm ID, 5 μm) (Supelcosil LC-18 column by Supelco), with precolumn (20 × 4.6 mm ID) filled with the same packing (Supelguard, Supelco) completed the analytical system. Two solvents, 0.01 M potassium phosphate buffer at pH 5.5 (solvent A) and methanol (solvent B), were used. The mobile phase gradient (% B) was 0 at 0 min, 10 at 10 min, 20 at 20 min, and 0 at 30 min. The next sample was injected after an additional 10 min. The flow rate was 1 mL/min, and the wavelength was 220 nm. We measured AOPP by the method described by Witko-Sarsat et al. (11), using spectrophotometry on a microplate reader. The AOPP were calibrated with chloramine-T solutions that absorb at 340 nm in the presence of potassium iodide. In test wells, 200 μL of plasma diluted 1:5 in PBS were distributed on a 96-well microtiter plate, and 20 μL of acetic acid were added. In standard wells, 10 μL of 1.16 M potassium iodide were added to 200 μL of chloramine–T solution (0–100 μmol/L) followed by 20 μL of acetic acid. The absorbance of the reaction mixture was immediately read at 340 nm on the microplate reader against a blank containing 200 μL of PBS, 10 μL of potassium iodide, and 20 μL of acetic acid. Because the absorbance of chloramine-T at 340 nm is linear up to 100 μmol/L, AOPP concentrations were expressed as μmol/L chloramine-T equivalents. TH production was measured with a d-ROMs Kit (Diacron srl, Italy). This method makes it possible to estimate the total amount of hydroperoxide present in a 10-μL blood sample by using a spectrophotometric procedure. Hydroperoxidic groups are attacked by the iron, decompartmentalized from transport proteins in 1 mL of acetate buffer at pH 4.8, to catalyze reactive oxygen metabolite formation by Fenton's reaction:MATH 1 MATH 2


The peroxy and alkoxy radicals produced, whose quantity is directly proportional to that of peroxides present in the plasma, are trapped chemically by 10 μL of chromogen (N,N-dyethyl-para-phenyl-diamine) in an electron-transfer process leading to the formation of the radical cation of this chromogen (A). MATH 3 MATH 4


The purple color resulting from this reaction over time was then monitored in an UV-VIS spectrophotometer (Perkin Elmer λ 16, Norwalk, CN) at 505 nm. The results were expressed in conventional arbitrary units, called Carr units. The value of 1 Carr unit is equal to a concentration of 0.08 mg/dl of hydrogen peroxide. Within-run variations were less than 2.6% and between-run variations less than 4.6%.
Several determinations, adding purified glutathione peroxidase with reduced glutathione in excess to the plasma sample, were performed to prove that the reaction was due to peroxy and alkoxy radical products. A decrease of more than 90% in the signal was observed.
The data (expressed as means ± SD) were analyzed for statistically significant differences by two-tailed t test for unpaired data and by linear correlation, using the SPSS/PC +4 statistical package (SPSS Inc, Chicago, IL).
RESULTS
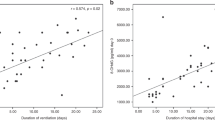
Hx, Xa, UA, TH, and AOPP plasma levels were significantly higher in hypoxic than normoxic newborns (Table 2 and Fig. 1). Similar results, but more statistically significant, were found in 15 of 39 hypoxic babies meeting stricter criteria of hypoxia (Hx: 4.87 ± 0.35 versus 1.13 ± 0.16 μg/mL, p < 0.0001; Xa: 1.42 ± 0.27 versus 0.56 ± 1.71 μg/mL, p < 0.0001; UA: 48.45 ± 4.44 versus 21.69 ± 3.3 μg/mL, p < 0.0001; TH: 226.11 ± 33.71 versus 45.72 ± 6.72 Carr U/L, p < 0.0001; AOPP: 229.45 ± 17.82 versus 125.88 ± 12.54 μmol/l, p < 0.0001). Statistically significant correlations were observed between TH and Hx (Fig. 2), AOPP and Hx (Fig. 3), and TH and AOPP plasma levels (Fig. 4).
DISCUSSION
FR generation during hypoxia recently was demonstrated by electron spin resonance spectroscopy of oxygen-free radicals during in utero hypoxia in fetal brain (15). The mechanism of damage from hypoxic-ischemic insult involves a series of events such as decrease in intracellular energy, decrease in membrane Na+,K+-ATPase activity, increase in intracellular Ca2+, and membrane lipid peroxidation (16, 17). The increased intracellular calcium concentration activates phospholipase A2 which results in the release of arachidonic acid and the subsequent generation of FR through the cyclooxygenase and lipoxygenase pathways of arachidonic acid metabolism. It can also activate nitric oxide synthetase, which leads to generation of nitric oxide and other FR such as hydroxyl radicals (18).
Increased degradation of ATP during hypoxia increases the substrate for xanthine oxidase reaction leading to increased FR generation (19). FR production may also occur in the presence of free iron in biological systems (20). Intracellular free iron may be released from ferritin under the conditions of decreased cellular high-energy compounds. This form of iron, unbound to transferrin in plasma or intracellularly by ferritin, is capable of inducing FR production by catalyzing the Fenton reaction. The presence of free iron in the erythrocytes and in plasma of newborn infants makes them more susceptible to non-protein-bound, iron-induced oxidative damage (9, 21). An additional source of FR generation may be phagocyte activation mediated by inflammatory reactions during hypoxia (22).
Whatever the origin, the generation of FR exposes the premature infant with a poorly developed antioxidant system to oxidative damage. The present study confirms increased FR generation during hypoxia. This was evident from the significantly higher levels of ATP degradation products, Hx, Xa, and UA, as expected in hypoxic preterm newborns, and of TH and AOPP as well (Fig. 1). TH concentrations are significantly correlated with Hx plasma levels. These data support the hypothesis that hypoxia induces increased FR generation and oxidative stress in the hypoxic preterm newborn. Such a conclusion is in accordance with our previous reports on high intraerythrocyte free iron levels and malondialdehyde plasma concentration in newborns with hypoxia (8, 9).
TH are good indices of overall FR attack, because they are indicative of intermediate oxidative products of lipids, peptides, and amino acids. Superoxide and hydroxyl radicals generated as the result of hypoxia are primarily extremely short-lived radicals that may not be amenable to ex vivo detection. Lipid and protein damage by FR exposure leads to lipid hydroperoxide generation from lipids and to carbonyl formation and protein hydroperoxide generation from proteins. Lipid and protein hydroperoxide, in the presence of traces of free iron, produces several secondary reactive radical species which can be measured collectively as organic hydroperoxide by a spectrophotometric assay. For measurement of oxygen radicals, the hydroperoxide assays lack specificity; however, they do provide a useful test to evaluate the overall oxidative stress in plasma in various clinical conditions (23). The significantly high TH plasma levels found in preterm hypoxic newborns are therefore indirect evidence of an increase in FR generation during hypoxia. The correlation between TH and Hx in plasma of preterm babies strongly suggests that the deeper the hypoxia, the greater the reactive oxygen metabolite production.
In contrast to lipids, the reactions of proteins with various oxidants have not been extensively studied in vivo, and therefore it was assumed that proteins were not particularly susceptible to FR damage. This assumption has been disproven in recent years, and it is now clear that amino acids, peptides, and proteins are vulnerable to attacks by a variety of FR and related oxidants (24, 25). Protein-FR interactions may have considerable significance in vivo, given that 10–50% of all antioxidant potential of human plasma challenged with peroxyl radicals seems to be due to proteins (26).
It has been demonstrated that altered protein molecules such as protein peroxides and protein-bound reducing moieties can act as “traps” for the chemical energy released by FR and initiate further radical chain reactions, thus enhancing the damage, as observed with lipid peroxides (27, 28). AOPP as terminal products of protein exposure to FR without oxidant properties are reliable markers of the degree of protein damage in oxidative stress. AOPP have been described as novel markers of protein oxidative stress in uremic patients (11). Biochemical characterization of AOPP in the plasma revealed two distinct peaks at 670 and around 70 kDa. Protein electrophoresis showed that the high-molecular-weight AOPP peak is attributable for the most part to albumin that appears to form aggregates, probably resulting from disulfide bridges and/or dityrosine cross-linking. The low-molecular-weight AOPP peak also contains albumin in its monomeric form (11).
We found higher AOPP plasma levels in hypoxic babies than in normoxic ones. This increased generation of oxidative protein products is correlated with the degree of hypoxia and with TH generation. The increased levels of AOPP and their correlation with ATP degradation products and TH generation indicate that plasma proteins, and particularly albumin, are involved in FR damage in preterm hypoxic babies. Plasma levels of AOPP are correlated with severity of chronic renal failure, malondialdehyde, and levels of pro-inflammatory cytokines IL-1β and TNF-α (12). These observations demonstrated that AOPP acts as a mediator of oxidative stress.
Varsila et al. (29) reported protein oxidation in preterm infants developing chronic lung disease. The present paper demonstrates, for the first time, protein oxidative damage in preterm hypoxic babies and confirms our previous reports on increased lipid peroxidation, phagocyte activation, and pro-inflammatory cytokines in vaginal delivery and emergency cesarean section, compared with elective cesarean section (30, 31).
Considering the above results, the increased FR generation during hypoxia sheds light on the mechanism of hypoxia-induced modification of molecular dysfunction. Although such changes do not necessarily indicate permanent damage, elucidation of these critical molecular processes could help in the development of future intervention strategies for minimizing hypoxia-related perinatal injury.
Abbreviations
- Hx:
-
hypoxanthine
- Xa:
-
xanthine
- UA:
-
uric acid
- AOPP:
-
advanced oxidation protein products
- TH:
-
total hydroperoxide
- FR:
-
free radical
References
Kelly FJ 1993 Free radical disorders of preterm infants. Br Med Bull 49: 668–678
Rice-Evans CA, Gopinathan V 1995 Oxygen toxicity, free radicals and antioxidants in human disease: biochemical implications in athero-sclerosis and the problem of premature neonates. Essay Biochem 19: 39–63
Pitkanen OM, Hallman M, Andersson S 1993 Correlation of free oxygen radial-induced lipid peroxidation with outcome in very-low-birthweight infants. J Pediatr 116: 760–764
Varsila E, Pitkanen O, Hallman M, Andersson S 1994 Immaturity-dependent free radical activity in premature infants. Pediatr Res 36: 55–59
Supnet MC, David-Cu R, Walther FJ 1994 Plasma xanthine oxidase activity and lipid hydroperoxide levels in preterm infants. Pediatr Res 36: 283–287
Schmidt H, Grune T, Muller R, Siems WG, Wauer RR 1996 Increased levels of lipid peroxidation products malondialdehyde and 4-hydroxynonenal after perinatal hypoxia. Pediatr Res 40: 15–20
Saugstad OD 1990 Oxygen toxicity in the neonatal period. Acta Paediatr 83: 692–695
Buonocore G, Zani S, Perrone S, Caciotti B, Bracci R 1998 Intraerythrocyte nonprotein-bound iron and plasma malondialdehyde in the hypoxic newborn. Free Radic Biol Med 25: 766–770
Buonocore G, Zani S, Sargentini I, Gioia D, Signorini C, Bracci R 1998 Hypoxia-induced free iron release in the red cells of newborn infants. Acta Paediatr 87: 77–81
Fu S-L, Dean RT 1997 Structural characterization of the products of hydroxyl-radical damage to leucine and their detection on proteins. Biochem J 324: 41–48
Witko-Sarsat V, Friedlander M, Capeillere-Blandin C, Nguyen-Khoa T, Thu Nguyen A, Zingraff J, Jungers P, Descamps-Latsca B 1996 Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int 49: 1304–1313
Witko-Sarsat V, Friedlander M, Nguyen-Khoa T, Capeillere-Blandin C, Thu Nguyen A, Canteloup S, Dayer J-M, Jungers P, Drueke T, Descamps-Latsca B 1998 Advanced oxidation protein products as novel mediators of inflammation and monocyte activation in chronic renal failure. J Immunol 161: 2524–2532
Davies MJ, Fu S, Dean RT 1995 Protein hydroperoxides can give rise to reactive free radicals. Biochem J 305: 643–649
American College of Obstetricians and Gynecologists 1994 Fetal distress and birth asphyxia. Washington, DC: ACOG. ACOG Committee Opinion 137
Maulik D, Numagami Y, Ohnishi TS, Mishra OP, Delivoria-Papadopoulos M 1998 Direct measurement of oxygen free radicals during in utero hypoxia in the fetal guinea pig brain. Brain Res 798: 166–172
McCord JM 1985 Oxygen derived free radicals in post-ischemic tissue injury. N Engl J Med 312: 159–163
Vannucci RC 1990 Experimental biology of cerebral hypoxia-ischemia: relation to perinatal brain damage. Pediatric Res 27: 317–326
Wolfe LS 1982 Eicosanoids: prostaglandins, thromboxanes, leukotrienes and other derivatives of carbon-20 unsaturated fatty acid. J Neurochem 38: 1–14
Saugstad OD, Gluck L 1982 Plasma hypoxanthine levels in newborn infants: a specific indicator of hypoxia. J Perinatal Med 10: 266–272
Gutteridge JMC, Rowley DA, Halliwell B 1981 Superoxide dependent formation of hydroxyl radicals in the presence of iron salts: detection of free iron in biological systems by using adriamycin-dependent degradation of DNA. Biochem J 199: 263–265
Moison RMW, Palinckx JJS, Roest M, Houdkamp E, Berger HM 1993 Induction of lipid peroxidation of pulmonary surfactant by plasma of preterm babies. Lancet 341: 79–82
Arnould T, Michiels C, Remacle J 1994 Hypoxic human umbilical vein endothelial cells induce activation of adherent polymorphonuclear leukocytes. Blood 83: 3705–3716
Cesarone MR, Belcaro G, Carratelli M, Cornelli U, De Sanctis MT, Incandela L, Barsotti A, Terranova R, Nicolaides A 1999 A simple test to monitor oxidative stress. Int Angiol 18: 127–130
Wolff SP, Garner A, Dean RT 1986 Free radicals, lipids and protein degradation. Trends Biochem Sci 11: 27–31
Davies JK 1987 Protein damage and degradation by oxygen radicals: general aspects. J Biol Chem 262: 9895–9901
Wayner DD, Burton GW, Ingold KU, Berclay LRC, Locke SJ 1987 The relative contributions of vitamin E, urate, ascorbate and proteins to the total peroxyl radical-trapping antioxidant activity of human blood plasma. Biochim Biophys Acta 924: 408–419
Simpson JA, Gieseg SP, Dean RT 1993 Free radical and enzymatic mechanisms for the generation of protein bound reducing moieties. Biochim Biophys Acta 1156: 190–196
Simpson JA, Narita S, Gieseg S, Gebicki S, Gebicki JM, Dean RT 1992 Long-lived reactive species on free-radical-damaged proteins. Biochem J 282: 621–624
Varsila E, Personen E, Andersson S 1995 Early protein oxidation in the neonatal lung is related to development of chronic lung disease. Acta Paediatr 84: 1296–1299
Buonocore G, Gioia D, De Filippo M, Picciolini E, Bracci R 1994 Superoxide anion release by polymorphonuclear leucocytes in whole blood of newborns and mothers during the peripartal period. Pediatr Res 36: 619–622
Buonocore G, De Filippo M, Gioia D, Picciolini E, Luzzi E, Bocci V, Bracci R 1995 Maternal and neonatal plasma cytokine levels in relation to mode of delivery. Biol Neonate 68: 104–110
Author information
Authors and Affiliations
Additional information
Supported by the Italian Ministry for University and Scientific-Technologic Research (MURST 40% and 60% funds).
Rights and permissions
About this article
Cite this article
Buonocore, G., Perrone, S., Longini, M. et al. Total Hydroperoxide and Advanced Oxidation Protein Products in Preterm Hypoxic Babies. Pediatr Res 47, 221 (2000). https://doi.org/10.1203/00006450-200002000-00012
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-200002000-00012
This article is cited by
-
Oxidative stress markers in neonatal respiratory distress syndrome: advanced oxidation protein products and 8-hydroxy-2-deoxyguanosine in relation to disease severity
Pediatric Research (2020)
-
Free radicals and neonatal encephalopathy: mechanisms of injury, biomarkers, and antioxidant treatment perspectives
Pediatric Research (2020)
-
A study of oxidative stress in neonates delivered through meconium-stained amniotic fluid
European Journal of Pediatrics (2017)
-
Increasing F2-isoprostanes in the first month after birth predicts poor respiratory and neurodevelopmental outcomes in very preterm infants
Journal of Perinatology (2016)
-
Oxidative stress ecology and the d-ROMs test: facts, misfacts and an appraisal of a decade’s work
Behavioral Ecology and Sociobiology (2016)