Abstract
Infantile hypertrophic pyloric stenosis (IHPS) is characterized by hypertrophy of the pyloric muscle. The growth of smooth muscle cells is regulated by several growth factors. Epidermal growth factor (EGF) and heparin-binding EGF-like growth factor are potent mitogens for smooth muscle cells. In the present study, we investigated immunohistochemical localization of EGF and EGF-related peptides and EGF mRNA expression in pyloric smooth muscle cells to determine whether the EGF family is involved in the process of pyloric muscle hypertrophy in IHPS. Pyloric muscle biopsy specimens were obtained at the time of pyloromyotomy from 10 patients with IHPS. Control material included 10 pyloric muscle specimens taken at autopsy from age-matched cases without evidence of gastrointestinal disease. Indirect immunohistochemistry was performed using the avidin-biotin-peroxidase complex method with anti-EGF, anti-EGF receptor, and anti–heparin-binding EGF-like growth factor antibody. In situ hybridization was performed using digoxigenin-labeled EGF-specific oligonucleotide probe. The pattern of immunoreactivity in pyloric muscle with EGF, EGF receptor, and heparin-binding EGF-like growth factor was similar in all specimens. There was a marked increase in EGF, EGF receptor, and heparin-binding EGF-like growth factor immunoreactivity and EGF mRNA expression in smooth muscle cells in pyloric circular and longitudinal muscle from patients with IHPS compared with control specimens. These data suggest that the up-regulated local synthesis of EGF and EGF-related peptides in pyloric muscle may play a critical role in the development of pyloric muscle hypertrophy in IHPS.
Similar content being viewed by others
Main
IHPS is characterized by hypertrophy of the pyloric muscle, causing pyloric-channel narrowing and elongation. Although the exact mechanisms responsible for smooth muscle hypertrophy are unknown, with progress in molecular biology, there is increasing evidence to suggest that the growth of SMCs is regulated by several growth factors (1–3). It has been well known that growth factors control cell proliferation and modulate other cellular functions by binding to specific high-affinity cell surface membrane receptors. Recent studies from our laboratory reported increased local synthesis of some potent mitogens of smooth muscle, such as IGF-I, PDGF-BB, and TGF-α, by the hypertrophic pyloric muscle (4–6). These findings suggested that the local production of peptide growth factors may be involved in the development of pyloric muscle hypertrophy in IHPS.
EGF is a polypeptide chain that belongs to an expanding group of growth factor ligands (7). EGF exerts a variety of biologic influences in many cell types. EGF has been reported to be a powerful mitogen and trophic agent in the gastrointestinal tract. It has been reported that EGF delays both gastric emptying and small intestinal motility in rats (8). Vinter-Jensen and colleagues (9, 10) have recently reported that systemic treatment with EGF induces SMC hyperplasia and hypertrophy in the urinary tract. The involvement of the EGF family in muscle cell growth has recently been suggested in vascular SMCs (11) and cardiac muscle cells (12), in which cell growth is associated with increased expression of the EGF-related ligand, HB-EGF. HB-EGF, discovered in 1991 (13), is a new member of the EGF family. Although HB-EGF, as well as EGF and TGF-α, binds to the EGF-R and activates EGF-R tyrosine kinase to induce various biologic effects, HB-EGF has been characterized as a much more potent mitogen for SMCs compared with other members of the EGF family. In the current study, we investigated immunohistochemical localization of EGF and EGF-related peptides and EGF mRNA expression in pyloric SMCs to determine whether the EGF family is involved in the process of pyloric muscle hypertrophy in IHPS.
METHODS
Tissue specimens.
Full-thickness pyloric muscle biopsy specimens were obtained at the time of pyloromyotomy from 10 patients with IHPS (age range, 24–76 d). Control material included 10 pyloric muscle specimens taken at autopsy from age-matched cases without evidence of gastrointestinal disease. All specimens were fixed in formalin and embedded in paraffin wax. Four-micrometer-thick sections were cut from paraffin blocks and mounted on polylysine-coated glass slides. The same specimens from each patient were used for immunohistochemistry and in situ hybridization.
Immunohistochemistry.
Formalin-fixed paraffin-embedded sections were immersed in a solution of 0.3% hydrogen peroxidase in methanol for 20 min to block endogenous peroxidase. Demasking technique, to expose antigenic site for immunohistochemistry of HB-EGF, was performed by heating in citric buffer twice for 5 min using a microwave oven. Immunohistochemistry was performed using the avidin-biotin-peroxidase complex method. Rabbit anti-human EGF polyclonal antibody was diluted at 1:200 (Chemicon International Ltd, Harrow, UK) in PBS with 10% normal goat serum. Also, goat anti-human HB-EGF polyclonal antibody (Santa Cruz Biotechnology Inc, Santa Cruz, CA) at 1:200 and mouse anti-human EGF-R MAb (Novocastra Laboratories Ltd, Newcastle, UK) at 1:20 were diluted with 10% rabbit serum. The sections were incubated in antiserum at 4°C overnight. Biotin-labeled goat anti-rabbit antibody for EGF and rabbit anti-goat antibody for HB-EGF at 1:400 dilution and rabbit anti-mouse for EGF-R antibody at 1:200 dilution (Dako-Patts, Glostrup, Denmark) in PBS for 1 h and avidin-biotin-peroxidase complex steps (30 min) were performed at room temperature. Peroxidase was visualized by diaminobenzidine and hydrogen peroxidase. Negative controls consisted of each case in which the primary antibody was omitted.
In situ hybridization.
The method used has been described previously (5). In situ hybridization using EGF-specific oligonucleotide probe (Biogenostic, Gottingen, Germany) was performed. The probe were labeled by DIG using DIG oligonucleotide tailing kit (Boehringer Mannheim, Mannheim, Germany). Formalin-fixed paraffin-embedded sections (4 μm) of the resected specimens were incubated for 30 min at 37°C in proteinase K in Tris buffer. The slides were placed in 0.4% paraformaldehyde in 1 × PBS for 20 min at 4°C. After tissue preparation, the sections were prehybridized with hybridization buffer at 37°C for 1 h. Then the slides were incubated 18 h at 37°C with hybridization buffer in which EGF probe was diluted to 100 ng/mL. Sections were processed for immunologic detection using alkaline phosphatase-conjugated anti-DIG serum. Nitroblue tetrazolium salt and 5-bromo-4-chloro-3-indolyl phosphate toluidinium salt were used as substrate. Sense probe was used as a positive control to confirm the sensitivity of the in situ hybridization method, and RNase treatment was performed to demonstrate the RNA specificity of the procedure. Negative controls consisted of each case in which the specific probe was omitted.
Calculation of results.
The immunoreactivity of EGF and EGF-related peptides and EGF mRNA expression on pyloric SMCs was graded independently by two of us (H.S., K.O.) without the knowledge of the patient's diagnosis as (−) indicating no expression; (±), weak; (+), moderate; and (++), strong. When the grades were not identical, sections were regraded and a consensus was reached.
The study was approved by the research council of our institution.
RESULTS
The results are summarized in Table 1. The pattern of immunoreactivity in pyloric muscle with EGF, EGF-R, and HB-EGF was similar in all specimens. There was a marked increase in EGF, EGF-R, and HB-EGF immunoreactivity and EGF mRNA expression in SMCs in pyloric circular and longitudinal muscle from patients with IHPS compared with control specimens.
EGF immunohistochemistry.
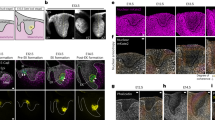
In normal control pyloric muscle, there was weak EGF immunoreactivity in SMCs in circular and longitudinal muscle (Fig. 1a). In contrast, there was strong immunoreactivity both in pyloric circular and longitudinal muscle in IHPS (Fig. 1b).
EGF-R immunohistochemistry.
In the control pyloric muscle, there was weak EGF-R immunoreactivity in SMCs in circular and longitudinal muscle (Fig. 2a). In contrast, there was strong immunoreactivity both in pyloric circular and longitudinal muscle in IHPS (Fig. 2b).
HB-EGF immunohistochemistry.
In pyloric muscle of normal control, there was weak HB-EGF immunoreactivity in SMCs in circular and longitudinal muscle (Fig. 3a). There was strong immunoreactivity both in pyloric circular and longitudinal muscle in IHPS (Fig. 3b).
In situ hybridization of EGF.
In the control pyloric muscle, there was very weak EGF mRNA expression in circular and longitudinal muscle (Fig. 4a), whereas strong EGF mRNA expression was detected in SMCs in pyloric circular and longitudinal muscle in IHPS (Fig. 4b).
DISCUSSION
Although the mechanisms responsible for smooth muscle hypertrophy are unknown, with progress in molecular biology (1–3), there is increasing evidence to suggest that the growth of SMCs is regulated by several growth factors (14, 15). IGF-I and PDGF-BB are potent SMC mitogens in vitro and act synergistically to stimulate SMC proliferation. IGF-I mediates the growth-promoting effects of PDGF in mesenchymal cells (16). IGF-I and PDGF have been shown to be produced by SMCs (17, 18), and their effects are mediated via their receptors (19). TGF-α is a growth regulatory peptide found in a wide range of embryonic and adult tissues. It has been recognized that TGF-α has a growth-promoting effect on vascular and visceral SMCs (20). We have previously reported increased expression of IGF-I, PDGF-BB, and TGF-α in hypertrophic pyloric muscle in IHPS (4–6). EGF is best known as a potent growth stimulator. It appears to play a critical role early in growth of cultured smooth muscle, in which its production is highest and its growth-promoting effects are greatest (20, 21). The present study demonstrated increased immunoreactivities of the EGF family and EGF-R, as well as increased mRNA expression of EGF, in hypertrophic pyloric muscle. These findings, together with our previously reported up-regulated expression of other smooth muscle factors (IGF-I, PDGF-BB, and TGF-α) in the hypertrophic pyloric muscle, suggest that the increased local synthesis of peptide growth factors in SMCs may play a critical role in the development of pyloric muscle hypertrophy in IHPS.
We recently investigated proliferative activity of SMCs in IHPS using MIB-1 immunohistochemistry, and we also measured SMC number and size using an image analyzer (22). Cell proliferation activity is an important indicator of cell hyperplasia. MIB-1 immunostaining has been reported to be suitable for assessing the proliferative activity, because it has less background staining and more uniform and stronger positive signals (23). We demonstrated that the percentage of MIB-1–positive pyloric cells is significantly higher in tissue from patients with IHPS than in normal control specimens, suggesting that increased proliferative activity of the SMCs plays an important role in the increasing pyloric muscle mass in IHPS. Proliferation and growth of cultured SMCs can be stimulated by a number of peptide growth factors such as IGF-I, PDGF, EGF, and TGF-β (2, 20, 24). It is thus possible that the local production of these peptide growth factors is involved in the development of pyloric muscle hypertrophy and hyperplasia in IHPS. It is interesting to speculate that the up-regulated local peptide growth factors may induce altered autocrine growth regulation in pyloric SMCs, contributing to the development of pyloric muscle hypertrophy and hyperplasia in IHPS.
There are several possible mechanisms to explain the development of smooth muscle hypertrophy. One hypothesis is that growth factors stimulate somatic growth (1). An alternative theory is that smooth muscle hypertrophy may be regulated in part by growth factors that alter the pattern of growth response to mitogens, resulting in incomplete growth stimulation (25). However, many questions regarding the functional role of growth factors in smooth muscle hypertrophy remain to be addressed. Further studies to elucidate a more precise molecular basis for pyloric muscle hypertrophy will lead us to the exact pathogenesis of this condition.
Abbreviations
- DIG:
-
digoxigenin
- EGF:
-
epidermal growth factor
- EGF-R:
-
EGF receptor
- HB-EGF:
-
heparin-binding EGF-like growth factor
- IHPS:
-
infantile hypertrophic pyloric stenosis
- PDGF-BB:
-
platelet-derived growth factor-BB
- SMC:
-
smooth muscle cell
- TGF-α:
-
transforming growth factor-α
References
Chen Y, Bornfeldt KE, Arner A, Jennische E, Malmqvist U, Uvelius B, Arnqvist HJ 1994 Increase in insulin-like growth factor-I on hypertrophying smooth muscle. Am J Physiol 266: E224–E229
Yamamoto M, Yamamoto K 1994 Growth regulation in primary culture of rabbit arterial smooth muscle cells by platelet-derived growth factor, insulin-like growth factor-I, and epidermal growth factor. Exp Cell Res 212: 62–68
Pfeifer TL, Chegini N 1994 Immunohistochemical localization of insulin-like growth factor (IGF-I), IGF-I receptor, and IGF binding proteins in the cardiovascular system. Cardiovasc Res 30: 281–289
Ohshiro K, Puri P 1998 Increased insulin-like growth factor-I and platelet-derived growth factor system in pyloric muscle in infantile hypertrophic pyloric stenosis. J Pediatr Surg 33: 378–381
Ohshiro K, Puri P 1998 Increased insulin-like growth factor-I mRNA expression in pyloric muscle in infantile hypertrophic pyloric stenosis. Pediatr Surg Int 13: 253–255
Shima H, Puri P 1999 Increased expression of transforming growth factor-α in infantile hypertrophic pyloric stenosis. Pediatr Surg Int 15: 198–200
Prigent SA, Lemoine NR 1992 The type 1 (EGFR-related) family of growth factor receptors and their ligands. Prog Growth Factor Res 4: 1–24
Shinohara H, Williams C, Yakabe T, Koldovsky O 1996 Epidermal growth factor delays gastric emptying and small intestine transit in sucking rats. Pedatr Res 39: 281–286
Vinter-Jensen L, Kirik D, Arner A, Nexo E, Uvelius B 1997 Acute contractile effects of epidermal growth factor on bladder smooth muscles. Scand J Urol Nephrol 31: 231–235
Vinter-Jensen L, Juhl CO, Dajani EZ, Nielsen K, Djurhuus JC 1997 Chronic systemic treatment with epidermal growth factor induces smooth muscle hyperplasia and hypertrophy in the urinary tract of mature Goettingen minipigs. Br J Urol 79: 532–538
Temizer DH, Yoshizumi M, Perrella MA, Susanni EE, Quertermouss T, Lee ME 1992 Induction of heparin-binding epidermal growth factor-like growth factor mRNA by phorbol ester and angiotensin II in rat aortic smooth muscle cells. J Biol Chem 267: 24892–24896
Perrela MA, Maki T, Prasad S, Pimental D, Singh K, Takahashi N, Yoshizumi M, Alali A, Higashiyama S, Kelly RA, Lee ME, Smith TW 1994 Regulation of heparin-binding epidermal growth factor-like growth factor mRNA levels by hypertrophic stimuli in neonatal and adult rat cardiac myocytes. J Biol Chem 269: 27045–27050
Higashiyama S, Abraham JA, Miller J, Fiddes JC, Klagsbrun M 1991 A heparin-binding growth factor secreted by macrophage-like cells that is related to EGF. Science 251: 936–939
Weinstein R, Stemmerma MB, Maciag T 1981 Hormonal requirements for growth of arterial smooth muscle cells in vitro : an endocrine approach to atherosclerosis. Science 212: 818–820
Raines E, Ross R 1991 Mechanisms of plaque formation: cellular changes and possible role of growth regulatory molecules. Atheroscler Rev 23: 143–152
Clemmones DR, Van Wyk JJ 1985 Evidence for a functional role of endogenously produced somatomedin like peptides in the regulation of DNA synthesis in cultured human fibroblast and porcine smooth muscle cells. J Clin Invest 75: 1914–1918
Libby P, Waener SJC, Salmone RN, Brinyi LK 1988 Production of platelet derived growth factor-like mitogen by smooth muscle cells from atheroma. N Engl J Med 318: 1493–1496
Cercek B, Fishbein MC, Forrester JS, Helfant RH 1990 Induction of insulin like growth factor I messenger RNA in rats aorta after balloon denudation. Circ Res 66: 1755–1760
Ultlrich A, Gray A, Tam AW, Yang-Feng T, Tsubokawa M, Collins C, Henzel W, LeBon T, Kathuria S, Chen F 1986 Insulin like growth factor I receptor primary structural determinants that define functional specificity. EMBO J 5: 2503–2512
Kuemmerle JF 1997 Autocrine regulation of growth in cultured human intestinal muscle by growth factors. Gastroenterology 113: 817–824
Schreiber AB, Winkler ME, Derynck R 1986 Transforming growth factor-α: a more potent angiogenic mediator than epidermal growth factor. Science 232: 1250–1253
Oue T, Puri P 1999 Smooth muscle cell hypertrophy versus hyperplasia in infantile hypertrophic pyloric stenosis. Pediatr Res 45: 853–857
Elias JM 1997 Cell proliferation indexes: a biomarker in solid tumors. Biotech Histochem 72: 78–85
Kirschenlohn HL, Metcalfe JC, Weissberg PL, Grainger DJ 1995 Proliferation of human aortic vascular muscle cells in culture is modulated by active TGF beta. Cardiovasc Res 29: 848–855
Owens GK, Geisterfer AA, Yang YW, Komoriya A 1988 Transforming growth factor-β induced growth inhibition and cellular hypertrophy in cultured vascular smooth muscle cells. J Cell 107: 771–780
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Shima, H., Ohshiro, K. & Puri, P. Increased Local Synthesis of Epidermal Growth Factors in Infantile Hypertrophic Pyloric Stenosis. Pediatr Res 47, 201 (2000). https://doi.org/10.1203/00006450-200002000-00009
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-200002000-00009
This article is cited by
-
New insights into the pathogenesis of infantile pyloric stenosis
Pediatric Surgery International (2009)
-
The development of fetal pylorus during the fetal period
Surgical and Radiologic Anatomy (2009)