Abstract
Acute right ventricular (RV) injury is commonly encountered in infants and children after cardiac surgery. Empiric medical therapy for these patients results from a paucity of data on which to base medical management and the absence of animal models that allow rigorous laboratory testing. Specifically, exogenous catecholamines have unclear effects on the injured right ventricle and pulmonary vasculature in the young. Ten anesthetized piglets (9–12 kg) were instrumented with epicardial transducers, micromanometers, and a pulmonary artery flow probe. RV injury was induced with a cryoablation probe. Dopamine at 10 μg/kg/min, dobutamine at 10 μg/kg/min, and epinephrine (EP) at 0.1 μg/kg/min were infused in a random order. RV contractility was evaluated using preload recruitable stroke work. Diastolic function was described by the end-diastolic pressure-volume relation, peak negative derivative of the pressure waveform, and peak filling rate. In addition to routine hemodynamic measurements, Fourier transformation of the pressure and flow waveforms allowed calculation of input resistance, characteristic impedance, RV total hydraulic power, and transpulmonary vascular efficiency. Cryoablation led to a stable reproducible injury, decreased preload recruitable stroke work, and impaired diastolic function as measured by all three indices. Infusion of each catecholamine improved preload recruitable stroke work and peak negative derivative of the pressure waveform. Dobutamine and EP both decreased indices of pulmonary vascular impedance, whereas EP was the only inotrope that significantly improved transpulmonary vascular efficiency. Although all three inotropes improved systolic and diastolic RV function, only EP decreased input resistance, decreased pulmonary vascular resistance, and increased transpulmonary vascular efficiency.
Similar content being viewed by others
Main
Acute RV injury is encountered in infants and children with congenital heart disease after cardiac surgery(1). Despite its frequent occurrence, the ideal therapeutic strategy to manage the young patient with acute RV systolic and/or diastolic dysfunction remains unknown. The management of infants and children with RV dysfunction in the intensive care unit is centered on providing adequate oxygen delivery through manipulations of the cardiac and respiratory systems. Techniques used to augment cardiac output after RV injury include inotropic support and manipulation of loading conditions(2, 3). Despite widespread use of inotropes, little is known about their detailed interactions on RV systolic function(2), RV diastolic function, and the pulmonary vasculature(4). Although these drugs may enhance intrinsic RV contractility, they may adversely affect RV afterload by increasing PVR(5).
Full understanding of RV function and its interaction with the pulmonary vasculature has been impeded by a lack of adequate animal models. Estimation of RV volume and, thus, characterization of ventricular function are made difficult by the complex geometry of the RV(6–8). To obtain a complete understanding of RV function and the effects of afterload on RV function, an accurate analysis of the pulmonary vasculature is essential. Previous descriptions of pulmonary hemodynamics have been limited to PA pressure, left atrial pressure, and mean PVR. A more complete understanding of the pulmonary vasculature, termed pulmonary vascular mechanics, can be achieved through Fourier transformation of the PA pressure and flow waveforms(9, 10). After Fourier transformation, pulmonary vascular impedance can then be described in terms of steady and pulsatile components. This in turn allows description of the RV energetics such as total hydraulic power and TVE(9, 11, 12).
To understand RV function and pulmonary vascular mechanics, the development of a stable animal injury model was required. Our initial hypothesis was that a model of RV injury using cryoablation would be stable, reproducible, and allow a reliable assessment of inotropic effects on RV systolic function, RV diastolic function, and pulmonary vascular mechanics. We subsequently hypothesized that infusions of commonly used exogenous catecholamines would improve RV systolic function and active relaxation, have no effect on RV chamber stiffness(13), and have variable effects on pulmonary vascular mechanics.
METHODS
Animal Preparation
Surgical procedures and animal care were in compliance with the guidelines established by the National Institutes of Health and the Institutional Animal Care and Use Committee of Duke University Medical Center. Ten young swine (9–12 kg) were premedicated with intramuscular acepromazine (1.0 mg/kg) and ketamine (20 mg/kg). Each animal then received an i.v. sodium thiopental bolus (25 mg/kg). The trachea was intubated, and the animal was placed on an SV300 ventilator (Siemens Inc.; Solno, Sweden) in the volume control mode. Tidal volume was set at 10 mL/kg, and positive end-expiratory pressure was set at 4 cm H2O. The FiO2 was maintained at 0.60 throughout the study to provide a situation of hyperoxia, thus avoiding the potential confounding variable of hypoxic vasoconstriction. The ventilatory rate was titrated to maintain normocapnia. (Arterial blood gas analyses were obtained to confirm a Pao2 greater than 200 torr and a PaCO2 35–40 torr before each data collection.) Vascular access was obtained in the femoral artery and vein under direct visualization. Anesthesia was maintained throughout the study with a continuous infusion of fentanyl (0.2 mg/kg/h). Pancuronium (1 mg/kg/dose) was intermittently administered for neuromuscular blockade.
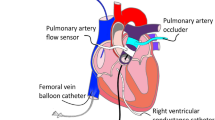
A median sternotomy was performed, the pericardium was incised, and a pericardial cradle was created. One-millimeter epicardial ultrasonic dimension transducers were sutured to the epicardium to measure the base to apex major axis diameter, the anterior to posterior minor axis diameter of the left ventricle, the left ventricular free wall to septal distance, and the RV free wall to septal distance. A septal crystal was placed into the interventricular septum and guided to lie as close to the right side of the septum as possible. An ultrasonic transit time flow probe (Transonic Systems Inc.; Ithaca, NY, U.S.A.) was placed around the pulmonary artery. Tapes were placed around both cavae. Micromanometer pressure transducers (MPC-500; Millar Instruments Inc.; Houston, TX, U.S.A.) were placed into the right and left ventricular cavities, left atrium, and PA at the level of the flow probe. Epicardial pacing wires were sutured onto the right atrium. Heart rate, cardiac rhythm, PA flow, and systemic arterial pressure were continuously monitored.
Protocol
Once instrumented, baseline (NML) data were acquired at steady state and during caval occlusion with ventilation paused at end-expiration. Stability of heart rate, blood pressure, arterial blood gas analysis, serum potassium, and serum calcium levels were confirmed before obtaining data. RV injury was then induced with a cryoablation probe placed on the RV epicardium (Frigitronics Inc.; Shelton, CT, U.S.A.) with care taken to avoid injuring the left anterior descending coronary artery. Each cryoablation (−50 to −70°C) was performed for 3 min per location along the RV outflow tract and the RV free wall from the base to the apex anteriorly and laterally. Sequential cryoablations were performed until RV injury occurred as defined by a 20% or greater decrease in systolic function as determined by the slope of PRSW. Each animal required between four and 10 cryoablations. Atrial pacing at physiologic rates was performed after the RV cryoablation process for all animals to overdrive junctional rhythms and bradyarrhythmias. Pacing was maintained during data collection. Data were obtained after injury was confirmed and stability of the animal preparation was assured (INJ).
DA at 10 μg/kg/min, DB at 10 μg/kg/min, and EP at 0.1 μg/kg/min were then infused in a random order. Steady state and caval occlusion data were acquired 20 min into each infusion. After the data collection for each inotrope, the infusion was discontinued. Due to the short half-life of the exogenous catecholamines, data were also acquired 20 min after discontinuing each infusion. (All animals returned to a stable condition of heart rate and blood pressure within this time period.) Data sets obtained before each drug infusion were used to represent the base-drug state. These data sets obtained between drug infusions were compared as described below to assure stability of the model.
At the conclusion of the protocol, each swine was sacrificed using an approved method, and the heart was removed. The RV free wall was excised, and the position of the septal crystal relative to the RV endocardial surface was documented. Left and right ventricular wall volumes were determined by saline displacement in a volumetric cylinder.
Data Acquisition
Analog flow, dimension, and pressure data were filtered by a 50-Hz low-pass circuit and digitized in real time at 200 Hz (caval occlusion) or 500 Hz (steady state) using an analog to digital converter (Scientific Solutions; Solon, OH, U.S.A.). Two data sets were obtained for each condition. Data were immediately reviewed for consistency and were stored for later analysis using custom software created in our laboratory.
Measurements
Pulmonary vascular mechanics.
Conventional measurements of pulmonary hemodynamics assume steady flow throughout the cardiac cycle. Because flow through the cardiovascular system is pulsatile, conventional analysis excludes the significant contribution of pulsatile flow to the understanding of pulmonary hemodynamics. This leads to an underestimation of RV energy requirements(12). We calculated the pulmonary vascular impedance spectrum through Fourier transformation of the pressure and flow waveforms to determine the effects of injury and catecholamine interventions on RV performance and the pulmonary vasculature. The concept of Fourier analysis is based on the general principle that periodic waves can be mathematically expressed as a sum of a series of pure sinusoidal harmonics. This Fourier series contains a zero frequency (mean) and oscillatory harmonic frequencies that are integer multiples of the original periodic waveform frequency, the so-called fundamental frequency. In biologic systems, the fundamental frequency is the heart rate. Each term in this series is defined by its amplitude and relative offset along the time axis (phase). The sum of these harmonics exactly replicates the original periodic waveform. The application of Fourier analysis of pulmonary arterial pressure and flow waveforms in vivo has been previously investigated(9, 10, 14). Fourier transformation of the pressure and flow data allows calculation of the impedance spectrum (input resistance and characteristic impedance) and RV energetics (total hydraulic power and TVE). Pressure and flow waveforms were averaged over 10–15 cardiac cycles on a point-to-point basis to yield a single averaged waveform for analysis.
Input resistance represents the total resistance to nonpulsatile RV outflow and was calculated as the ratio of mean PA pressure to flow(12). PVR is the component of input resistance attributable to the pulmonary vasculature and is calculated similarly to input resistance using the transpulmonary mean pressure difference (mean PA pressure minus left atrial pressure) instead of PA pressure. Characteristic impedance is defined as the impedance in the absence of reflected waves and correlates directly with the elastic properties and inversely with the cross-sectional area of the vascular bed. An estimate of characteristic impedance was made by averaging the input impedance values between 2 and 11 Hz.
RV energetics.
RV total hydraulic power is the sum of both steady and oscillatory components. It is derived from the impedance spectrum and the Fourier series of the flow waveforms(9). Total power as calculated in this study is the amount of work per unit time dissipated in the passage of blood across the pulmonary bed. It does not, however, represent the total power output of the RV, which necessarily includes a component to overcome the viscoelastic impedance of the RV myocardium. TVE is defined as the quotient of mean pulmonary blood flow and RV total hydraulic power(11, 15). Efficiency reflects the ease with which blood flows through the pulmonary vasculature. A greater TVE reflects increased flow through the pulmonary circuit for a given amount of RV total power dissipated within the blood vessels.
Systolic and diastolic function.
To evaluate systolic and diastolic function, pressure-volume loops were generated. RV volume estimation was performed using the ellipsoidal shell subtraction technique described by Feneley et al.(8). PRSW(16) was used to estimate RV systolic function. A simple linear regression equation was fit to the relationship of stroke work and end-diastolic volume obtained during caval occlusion, the slope of which describes PRSW. This index has been shown to be reproducible in the RV and relatively independent of the effects of preload and afterload(16, 17). RV chamber stiffness was characterized by the EDPVR. The monoexponential relationship P = beKcV was fit using least-squares regression to the end-diastolic pressure versus volume curve generated during vena cava occlusions(18, 19). In this equation, P is the diastolic pressure, V represents ventricular volume, Kc is the stiffness parameter, and b is a scaling constant. The stiffness parameter is inversely related to diastolic compliance with a higher number reflecting decreased compliance. Early diastole was characterized by the dP/dtmin. The rapid filling phase of diastole was quantified using the time derivative of the volume model to yield an estimate of the peak ventricular filling rate [Qpk = d(Volume)/dt].
Data Analysis
Hemodynamic measurements, PRSW, EDPVR, and the stroke volume/ejected volume ratio were compared among the various interventional states using a linear regression implementation of repeated measures ANOVA. Analysis of routine hemodynamic variables, pulmonary vascular mechanics, RV energetics, and RV systolic and diastolic function allowed for characterization of the RV injury. Comparisons were made among the following conditions: baseline (NML), injured (INJ), and base-drug states (BaseDA, BaseDB, BaseEP). Significant differences among these variables between the baseline and injured states supported that injury had occurred. Stability of the injury model was confirmed by analyzing these parameters among the injured and base-drug states with respect to order in the study. This would detect any possible degradation or spontaneous improvement in the injury model over time. Catecholamine effects (drug versus base-drug) and interdrug comparisons were tested using the differences between each drug and its respective base-drug state.
Significance tests for the peak negative pressure derivative (dP/dtmin) and Qpk were performed using heart rate as a time-varying covariate. Least squares means for the different interventional states were calculated using linear regression, and tests of statistical significance were carried out using multiple paired t tests with Bonferroni correction. A p value < 0.05 was considered statistically significant.
Volume Model
To confirm that injury did not significantly alter dynamic volume estimation, the slope and correlation coefficient (r2) of the linear relationship between the stroke volume calculated from the shell-subtraction technique and the ejected volume measured with the flow probe during caval occlusion were examined. The stroke volume was defined as the change in calculated volume from end-diastole to end-systole. Ejected volume was described as the area under the PA flow curve throughout the cardiac cycle.
RESULTS
Injury Model and Stability
Hemodynamics, pulmonary vascular mechanics, and RV energetics.
Cardiac output as measured by the PA flow probe was reduced with cryoablation from a baseline value of 880 ± 259 mL/min to an injured value of 649 ± 235 mL/min (p < 0.05). Cryoablation increased mean PA pressure (12.1 ± 2.5 versus 15.2 ± 3.4 mm Hg, p < 0.05), increased input resistance (1145 ± 256 versus 2002 ± 598 dyne·s·cm-5, p < 0.05), and decreased RV total power (29.9 ± 14.8 versus 25.6 ± 13.5 mW, p < 0.05) (Table 1). Similarly, TVE tended to decrease with injury, although this change was not statistically significant. Thus, flow and total power fell whereas PA pressure, PVR, and input resistance increased with RV injury.
Systolic and diastolic function.
Figure 1A shows an example of PRSW slopes in the baseline and injured states. Cryoablation produced a mean reduction of 39% in RV contractility as measured by PRSW slope (22.8 ± 7.8 versus 13.9 ± 4.1 erg·103·mL-1, p < 0.05) (Table 1). RV chamber stiffness, as measured by Kc, more than doubled with cryoablation (0.11 ± 0.05 versus 0.23 ± 0.07 mm Hg/mL, p < 0.05), consistent with a decrease in ventricular compliance. The dP/dtmin became more positive (−225 ± 46 versus-199 ± 43 mm Hg/s, p < 0.05), reflecting impaired active relaxation after cryoablation. Rapid filling, as measured by the Qpk, similarly decreased considerably after injury (71 ± 17 versus 45 ± 15 mL/s, p < 0.05). Thus, all three indices of diastolic function reflected injury.
RV PRSW. (A) PRSW for baseline (NML), injured (INJ), and base-drug states (BaseDA, BaseDB, BaseEP) demonstrating the significant reduction in systolic function after injury and the similarity of the injured state with the base-drug states. The injured and base-drug states were not significantly different from one another. * p < 0.05 vs Injury. (B) PRSW for base-drug (BaseDA, BaseDB, BaseEP) and drug (DA, DB, EP) states demonstrating the significant improvement in RV systolic function with each of the inotrope infusions. * p < 0.05 vs base-drug state. BaseDA indicates base-dopamine;BaseDB, base-dobutamine;BaseEP, base-epinephrine.
Stability of the model.
No time differences were found among the injured and base-drug states for systolic function (PRSW), diastolic function (EDPVR, dp/dtmin, Qpk), RV total power, TVE, mean PA pressure, input resistance, PVR, or cardiac output (PA flow). Each of these variables did not change over the time course of the study, confirming stability of the model. Also, heart rate and left atrial pressure did not change significantly among the uninjured, injured, or base-drug states. The consistency in PRSW among the base-drug states is shown in Figure 1A.
Inotropic Interventions
Hemodynamics.
PA blood flow significantly increased with each catecholamine infusion. Despite an increase in flow, EP decreased PA pressure (14.9 ± 3.2 versus 12.7 ± 2.4 mm Hg, p < 0.05) and was the only drug to significantly alter PA pressure from the base-drug state (Table 2). A decrease in PA pressure during infusion of EP coincided with a reduction in PVR (1469 ± 626 versus 945 ± 535 dyne·s·cm-5, p < 0.05). DB also decreased PVR (1377 ± 532 versus 1085 ± 685 dyne·s·cm-5, p < 0.05); however, no changes in PA pressure occurred.
Pulmonary vascular mechanics and RV energetics.
Input resistance fell with both EP (2090 ± 733 versus 1416 ± 566 dyne·s·cm-5, p < 0.05) and DB (2024 ± 627 versus 1642 ± 503 dyne·s·cm-5, p < 0.05) yet remained essentially unchanged with DA (1981 ± 603 versus 1939 ± 540 dyne·s·cm-5, p = NS) (Table 2). Characteristic impedance did not significantly change during infusions of catecholamines. This is anticipated because catecholamines are not expected to alter the elastic properties of the proximal vasculature. Total hydraulic power increased during infusion of DA (22.5 ± 11.0 versus 33.2 ± 22.8 mW, p < 0.05), DB (24 ± 9.6 versus 33.0 ± 16.7 mW, p < 0.05), and EP (24.8 ± 10.0 versus 28.8 ± 12.9 mW, p < 0.05). Infusion of EP increased TVE from baseline (mean difference = 2.7 L/W·min, p < 0.05), whereas TVE decreased during infusion of DA (mean difference = −3.9 L/W·min, p < 0.05). Administration of DB did not significantly affect TVE (mean difference = −1.1 L/W·min).
Systolic and diastolic function.
Infusions of each catecholamine led to a significant increase in RV contractility as measured by the PRSW slope (Fig. 1B). Although the increase in intrinsic contractility during administration of DB appeared to be greater than the effect seen by both DA and EP, this did not achieve statistical significance (p = 0.06). The stiffness parameter, Kc, fell during administration of DA, suggesting an improvement in the lusitropic state of the ventricle (Table 2). Active relaxation as measured by dP/dtmin significantly improved during infusion of each catecholamine. Both DA and DB demonstrated greater increases in dP/dtmin compared with EP. Peak ventricular filling rate did not change during any drug infusion.
Volume Model
The slopes, correlation coefficients, and intercepts of the linear regression for stroke volume versus ejected volume during caval occlusion were compared during baseline, injured, and base-drug states to determine whether the injury altered volume estimation. The slopes varied from 0.70 to 0.76 with r2 values from 0.80 to 0.84. The relationship between stroke volume and ejected volume as well as the correlation coefficients and intercepts were consistent. No statistical differences were found.
DISCUSSION
Injury Model and Stability
We have developed a stable and reproducible model of RV injury in young swine that induces both systolic and diastolic dysfunction. Cryoablation leads to a profound decrease in the inotropic state of the RV as measured by PRSW, which remained stable over the course of the study. Similarly, injury led to increased chamber stiffness, impaired active relaxation, and diminished Qpk. As with systolic function, all diastolic indices remained consistent during the base-drug states, further confirming stability of the injury model.
As noted with ventricular function, PA flow, PVR, input resistance, PA pressure, and RV total power all significantly changed after injury and remained stable throughout the study duration. The decrease in PA flow seen with injury coincided with a rise in PVR and PA pressure. This elevation in PVR and PA pressure may be secondary to a release of endogenous catecholamines that may have occurred as a result of ventricular injury. Because our study demonstrated that EP decreased PVR, one might expect similar findings with RV injury alone. However, in the situation of RV injury, without exogenous catecholamine administration, the relative amounts as well as the varying types (e.g. norepinephrine) of the different endogenous catecholamines released are not known, and, thus, the findings may be unpredictable.
To assure that cryoablation did not alter the consistency of RV volume estimation, stroke volume data obtained from end-diastolic and end-systolic volumes of the shell subtraction model were compared with the ejection volume calculated as the area under the PA flow waveform. Good correlation and consistent slopes throughout the experiment support the assumption that the volume model did not significantly change due to the injury imposed on the RV. Thus, the differences seen among volume-dependent indices were likely due to true differences in those indices and not to alterations in the volume estimate caused by the injury.
Catecholamine Administration
The effects of inotropes on RV function have been partially studied in clinical and laboratory settings. Friedman et al.(20) studied RV systolic and diastolic function in patients undergoing cardiac catheterization and noted that DB significantly improved cardiac performance. However, load-independent indices of systolic function were not evaluated, and DB doses varied. In a study by Krams et al.(21), an occlusion of the left anterior descending coronary artery in swine showed a significant decrease in the slope of the end-systolic pressure-segment length relationship and external work of the RV after ischemia. A DB infusion led to a return to baseline values. Other indices of systolic function were not evaluated and transpulmonary vascular mechanics were not interpreted, making it difficult to eliminate the effects of loading conditions. Greyson et al.(2) studied the effects of DB on dogs with and without RV free wall ischemia. His study illustrated a significant increase in segmental wall shortening and peak dP/dtmax after administration of DB.
Our study demonstrated that DA, DB, and EP improved RV systolic function and active relaxation of the RV (diastolic function). Although DB appeared to increase systolic function as measured by PRSW slope to a greater degree than either DA or EP, this did not achieve statistical significance (p = 0.06). All three catecholamines improved active relaxation as demonstrated by an increase in dP/dtmin. It is known that dP/dtmin is afterload dependent and correlates directly with ventricular afterload(22, 23). In this study, DB and DA produced a greater improvement in dP/dtmin than did EP. Although the increase of dP/dtmin produced by EP may have been mitigated by the profound decrease in input resistance with EP, the differences in dP/dtmin improvement among the three drugs did not change appreciably with the addition of input resistance as a covariate in the statistical model.
Peak ventricular filling rate did not change during any exogenous catecholamine infusion. This may be due to the mechanism of injury, acute myocardial necrosis, which may irreversibly stiffen the ventricle. This in turn may impair elastic recoil, which will diminish the pressure gradient between the right atrium and right ventricle with a subsequent decrease in the Qpk. Courtois et al.(24) and Ishida et al.(25) have demonstrated this in the left ventricle. Although increases in active relaxation improve rapid filling(26), the improvement in active relaxation seen in our study may not have been sufficient to counteract the above mentioned variables that serve to decrease rapid filling. Additionally, the limitations of accuracy or inherent sensitivity of the volume model may have led to an inability to detect a difference in Qpk(27).
It is generally believed that adrenergic agonists do not have a significant effect on chamber stiffness(13). The decrease in the stiffness parameter, Kc, noted during infusion of DA suggests it may improve RV chamber stiffness. Although the change in Kc was statistically significant, the absolute change was small and probably not clinically significant. The apparent improvement in chamber stiffness is not due to the improvement in active relaxation as measured by dP/dtmin, as all three adrenergic agents improved active relaxation by this method, and neither DB nor EP improved RV chamber stiffness. In fact, there is evidence that administration of EP to the postbypass newborn piglet increases left ventricular chamber stiffness(28).
Effect of catecholamines on hemodynamics and pulmonary vascular mechanics.
Previously, EP has been shown to have variable effects on the pulmonary vasculature. Consistent with our findings, other investigators have reported a significant decrease in PVR during EP infusion in piglets(29, 30). Doses in these studies ranged from 0.2 to 15 μg/kg/min. Royster et al.(31) demonstrated that PVR did not change with the use of low-dose EP (0.03 μg/kg/min) in postoperative coronary artery bypass graft patients. No data exist in infants or children on the effects of EP on pulmonary vascular reactivity. The result that EP significantly decreased PVR may be considered unexpected given the α-agonist properties of EP. However, at the dose of 0.1 μg/kg/min, the relative effects of β-receptor stimulation may be greater than α-receptor stimulation. A dose titration study would be needed to elaborate further.
In our model, DB significantly decreased input resistance and PVR. Also, PA flow significantly increased with no appreciable change in PA pressure. This is consistent with the findings by Furman et al.(32) who showed that DB (2–20 μg/kg/min) prevented hypoxic pulmonary vasoconstriction and decreased PVR in swine. Studies performed in children have demonstrated conflicting results. Berner et al.(33) showed a decrease in PVR in children after cardiac surgery with DB at 5 μg/kg/min. Driscoll et al.(4) infused DB (2.0 and 7.75 μg/kg/min) in children with congenital heart disease during cardiac catheterizations without a change in PVR.
TVE is defined as the quotient of the mean PA blood flow and the RV total power. TVE couples the ease with which blood passes through the pulmonary vasculature with the ability of the RV to generate flow(11, 15). EP was distinguished from the other catecholamines by significantly improving TVE (Table 3). DA, however, led to a statistically significant decrease in efficiency. Although all inotropes increased PA flow and total power, the relative increase in flow per unit power was greater for EP than for DA and DB. Thus, EP had the largest increase in PA flow relative to the increase in RV total power. This increase in efficiency is important in that it demonstrates that the increase in RV total power, which my reflect RV myocardial oxygen requirements, is offset by an even greater increase in PA blood flow. Also, EP increased efficiency, RV total power, and PA flow at a lower PA pressure. This is clinically significant because increased PA blood flow correlates with increased overall cardiac output, assuming a shunt lesion is not present. The decrease in RV total power is related to the decreased afterload from the vasodilatory contribution of EP as demonstrated by the decrease in input resistance and PVR. This finding may be explained by a greater β-agonist effect than α-agonist effect at an EP dose of 0.1 μg/kg/min in swine.
Overall, the combination of physiologic factors seen in our study suggests that EP may be more advantageous to the acutely injured RV. Further studies are necessary to determine whether the effects seen in swine are unique to their species or if there is indeed a benefit to the use of EP in the postoperative child with acute RV injury.
Study Limitations
The ventricular dysfunction created in this model is unique and not identical to the clinical setting of postoperative congenital heart disease. An exact model of ventricular dysfunction seen with congenital heart disease would require swine with such disease or surgically created heart disease. Also, such a model would ideally include the macro- and microvascular changes unique to congenital heart disease involving the right heart, such as tetralogy of Fallot. Repair using cardiopulmonary bypass would be required. Such experiments are relatively prohibitive because of the prolonged time required, the high expected mortality rate, and the difficulty in controlling the degree of injury. Cryoablation is effective, leads to a reproducible RV injury, and is relatively easy to perform with a low associated mortality (15%).
Other potential limitations of this study are that the inotropes were administered for short durations after the creation of RV injury and only one infusion rate was used for each catecholamine. Further explanation of the findings of this study may have been possible if the inotropes were administered to a control group with normal RV function or if several different doses of inotropes were used. However, this study was designed to mimic the common clinical scenario, and, thus, the inotropes were only administered after the creation of RV dysfunction. Further studies are needed in which the rate of inotrope administration is altered.
The volume-estimation method used during this study was the RV shell subtraction technique. This methodology does not accurately represent absolute RV volumes because a single dimension is measured across the RV and variation in transducer placement can introduce large variability of the volume estimates between animals. However, the repeated measures design of this study reduced the impact of between-animal variation, and the regression analysis of calculated stroke volume versus measured ejected volume supported the use of this volume-estimation method.
Summary
Cryoablation resulted in a stable reproducible RV injury model. All three inotropes studied improved RV systolic function and active relaxation of the RV. Although both EP and DB decreased input resistance and PVR, only EP increased overall TVE. This model may be applicable to other medical or surgical interventions for the infant or child after congenital heart surgery. Clinical studies are necessary in the pediatric patient to determine whether the acutely injured RV and pulmonary vasculature would respond similarly to exogenous catecholamine administration.
Abbreviations
- RV:
-
right ventricle
- DA:
-
dopamine
- DB:
-
dobutamine
- EP:
-
epinephrine
- PRSW:
-
preload recruitable stroke work
- EDPVR:
-
end-diastolic pressure-volume relationship
- Kc:
-
right ventricular chamber stiffness
- dP/dtmin:
-
peak negative value of the RV pressure waveform derivative
- Qpk:
-
peak filling rate
- TVE:
-
transpulmonary vascular efficiency
- PA:
-
pulmonary artery
- PVR:
-
pulmonary vascular resistance
References
Hijazi Z, Hillenbrand W 1992 The right ventricle in congenital heart disease. Cardiol Clin 10: 91–110.
Greyson C, Jorge G, Maria M, Schwartz G 1995 Effects of inotropic stimulation on energy metabolism and systolic function of the ischemic right ventricle. Am J Physiol 268: H1821
Imai T, Saitoh K, Kani H, Fujita T, Murata K 1992 Combined dose ratios of dopamine and dobutamine and right ventricular performance after cardiac surgery. Chest 101: 1197–1201.
Driscoll D, Gillette P, Duff D, Nihill M, Gutgessel H, Vargo T, Mullins C, McNamara D 1979 Hemodynamic effects of dobutamine in children. Am J Cardiol 43: 581–585.
Abdul-Rasool I, Chamberlain J, Swan P, Ward D 1987 Cardiorespiratory and metabolic effects of dopamine and dobutamine infusion in dogs. Crit Care Med 15: 1044–1050.
Reedy T, Chapman C 1963 Measurement of right ventricular volume by cineangiofluorography. Am Heart J 66: 221–225.
Reiter S, Rumberger J 1986 Precision of measurements of right and left ventricular volume by cine computed tomography. Circulation 74: 890–900.
Feneley MP, Elbeery JR, Gaynor JW, Gall S, Davis J, Rankin S 1990 Ellipsoidal shell subtraction model of right ventricular volume. Circ Res 67: 1427–1436.
Milnor W, Bergel D, Bargaimer J 1966 Hydraulic power associated with pulmonary blood flow and its relation to heart rate. Circ Res 19: 467–480.
Meyers C, Prurt C, D'Amico T, Smith P, Sabiston D, Van Trigt P 1992 Pulmonary arterial impedance after single lung transplantation. J Surg Res 52: 459–465.
Hillman N, Meliones J, Black D, Craig D, Cheifetz I, Smith P 1995 In acute lung injury, inhaled nitric oxide improves ventilation-perfusion matching, pulmonary vascular mechanics and transpulmonary vascular efficiency. J Thor Cardiovasc Surg 110: 593–600.
Milnor WR 1990 Hemodynamics, 2nd Ed. Williams and Wilkins, Baltimore, pp 127–131, 209
Kass D, Wolff M, Ting C, Liu C, Chang M, Lawrence W, Maughan W 1993 Diastolic compliance of hypertrophied ventricle is not acutely altered by pharmacologic agents influencing active processes. Ann Intern Med 119: 466–473.
Attinger E, Anne A, McDonald D 1966 Use of Fourier series for the analysis of biological systems. Biophys J 6: 291–304.
Cheifetz IM, Craig DM, Kern FH, Black DR, Hillman ND, Greeley WJ, Ungerleider RM, Smith PK, Meliones JN 1996 Nitric oxide improves transpulmonary vascular mechanics but does not change intrinsic right ventricular contractility in an acute respiratory distress syndrome model with permissive hypercapnea. Crit Care Med 24: 1554–1561.
Glower D, Spratt J, Snow N, Kabas J, Davis J, Olsen C, Tyson G, Sabiston D, Rankin J 1985 Linearity of the Frank-Starling relationship in the intact heart: the concept of preload recruitable stroke work. Circulation 71: 994–1009.
Karunanithi MK, Michniewicz J, Copeland SE, Feneley MP 1992 Right ventricular preload-recruitable stroke work, end-systolic pressure-volume, and dP/dtmax-end-diastolic volume relations compared as indexes of RV contractile performances in conscious dogs. Circulation 70: 1169–1179.
Glantz S, Parmley W 1978 Factors which affect the diastolic pressure-volume curve. Circ Res 42: 171–180.
Dell'Italia L, Walsh R 1988 Right ventricular diastolic pressure-volume relations and regional dimensions during acute alterations in loading conditions. Circulation 77: 1276–1282.
Friedman B, Lozner E, Curfman G, Herzberg D, Rolett E 1984 Characterization of the human right ventricular pressure-volume relation: effect of dobutamine and right coronary artery stenosis. J Am Coll Cardiol 4: 999–1005.
Krams R, Soei L, McFalls E, Winkler Prins E, Sassen L, Verdouw P 1993 End-systolic pressure length relations of stunned right and left ventricles after inotropic stimulation. Am J Physiol 265: H2099
Weisfeldt M, Scully H, Frederiksen J, Rubenstein J, Pohost G, Beierholm E, Bello A, Daggett W 1974 Hemodynamic determinants of maximum negative dP/dt and periods of diastole. Am J Physiol 227: 613–621.
Starling M, Montgomery D, Mancini G, Walsh R 1987 Load independence of the rate of isovolumic relaxation in man. Circulation 76: 1274–1281.
Courtois M, Kovacs S, Ludbrook P 1988 Transmitral pressure-flow velocity relation: importance of regional pressure gradients in the LV during diastole. Circulation 78: 661–671.
Ishida Y, Meisner J, Tsujioka K, Gallo J, Yoran C, Frater R, Yellin E 1986 Left ventricular filling dynamics: influence of LV relaxation and left atrial pressure. Circulation 76: 187–196.
Choong C, Abascal V, Thomas J, Guerrero J, McGlew S, Weyman A 1988 Combined influence of ventricular loading and relaxation on the transmitral flow velocity profile in dogs measured by Doppler echocardiography. Circulation 78: 672–683.
Lew W 1989 Evaluation of left ventricular diastolic function. Circulation 79: 1393–1397.
Caspi J, Coles J, Benson L, Herman S, Augustine J, Tsao P, Brezina A, Kolin A, Wilson G 1993 Effects of high plasma epinephrine and Ca2+ concentrations on neonatal myocardial function after ischemia. J Thorac Cardiovasc Surg 105: 59–67.
Barrington K, Chan W 1993 The circulatory effects of epinephrine infusion in the anesthetized piglet. Pediatr Res 33: 190–194.
Meadow W, Rudinsky B, Strates E 1986 Selective elevation of systemic blood pressure by epinephrine during sepsis-induced pulmonary hypertension in piglets. Pediatr Res 20: 872–875.
Royster R, Butterworth J, Prielipp R, Zaloga G, Lawless S, Spray B, Kon N, Wallenhaupt S, Cordell A 1993 Combined inotropic effects of amrinone and epinephrine after cardiopulmonary bypass in humans. Anesth Analg 77: 662–672.
Furman W, Summer W, Kennedy T, Sylvester J 1982 Comparison of the effects of dobutamine, dopamine, and isoproterenol on hypoxic pulmonary vasoconstriction. Crit Care Med 10: 371–374.
Berner M, Rouge J, Friedli B 1983 The hemodynamic effect of phentolamine and dobutamine after open-heart operations in children. Ann Thorac Surg 35: 643–650.
Author information
Authors and Affiliations
Additional information
Supported by a Children's Miracle Network Telethon Grant from Duke Children's Hospital.
Rights and permissions
About this article
Cite this article
McGovern, J., Cheifetz, I., Craig, D. et al. Right Ventricular Injury in Young Swine: Effects of Catecholamines on Right Ventricular Function and Pulmonary Vascular Mechanics. Pediatr Res 48, 763–769 (2000). https://doi.org/10.1203/00006450-200012000-00011
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-200012000-00011