Abstract
It is well known that the sex difference in body growth at puberty is modulated by a complex interplay between sex steroids and somatotropic axis; however, the exact role played by sex steroids remains a matter of controversy. The aim of this study was to assess the mechanisms by which sex steroids regulate body growth during pubertal development. Flutamide, a non–steroid-blocking androgen receptor, was subcutaneously administered to 30-d-old male Wistar rats for 4 wk. The blockade of the androgen receptor led to a marked elevation in serum testosterone and an increment in serum estradiol. Flutamide administration decreased body weight gain, serum IGF-I levels, hepatic IGF-I mRNA, and GH receptor mRNA content. There were no significant changes in serum GH concentration, pituitary GH reserve, and pituitary GH mRNA content. Flutamide lowered hypothalamic somatostatin mRNA content and augmented hypothalamic immunoreactive somatostatin stores, but did not alter hypothalamic immunoreactive GH-releasing factor stores. Our findings indicate that during pubertal development of the male rat, the imbalance between androgen and estrogen actions determines an abnormal somatic growth, which is at least partly exerted through the peripheral or hepatic modification of the somatotropic axis that occurs under the high or exclusive action of estrogens. Potential implication of coincident sex-specific regulated mode of pulsatile GH secretion cannot be excluded from this random serum GH sample study.
Similar content being viewed by others
Main
Sexual dimorphism in pubertal growth spurt is controlled mainly through complex interactions between GH, IGF-I, and gonadal steroids. Apparently, androgens stimulate somatic growth rate in pubertal male rodents inasmuch as orchidectomy in prepubertal male animals results in a decreased body weight gain or linear growth (1). However, orchidectomy in the male produces a situation of low androgens and low estrogens, and it is well known that estrogens also exert some effect on somatic growth (2). The mode of pulsatile GH secretion, which increases during puberty in both sexes (3), is also sexually dimorphic (4). Estrogen administration feminizes the pulsatile GH secretory pattern in male rats (5), and the nonaromatizable DHT masculinizes the GH secretory rate in female rats (6). In addition to a potential direct action, GH increases somatic growth through the induction of liver IGF-I gene expression and secretion (7). Several studies indicate that estrogens directly inhibit liver IGF-I gene expression and its circulating serum levels (2), whereas androgens have no direct action on IGF-I regulation (8), suggesting that the increased serum IGF-I levels observed in rats with an unbalanced increased action of androgens might be mediated through enhancement of GH production. Recent studies suggest that the stimulatory effect of testosterone on the somatotropic axis may occur through its aromatization to estradiol (9).
When comparing female rats with male rats, the lower amplitude, higher frequency, and more irregularity of GH pulses determines a nearly continuous mode of GH secretion as analyzed by approximate entropy statistics (10, 11). However, the higher mean GH levels that result from this secretory pattern in female rats are associated with a lower somatic growth rate, suggesting that sex steroids modulate GH responsiveness in peripheral tissues. GH, and particularly the mode of its pulsatile secretion, up-regulates the expression of its receptor, whose mRNA content rises during puberty when the increase in GH production and amplitude of its pulses takes place (12). Although it is clear that GH is an essential factor for steroid regulation of GHR gene expression (13, 14), some controversy on the direct effect of gonadal steroids on GHR remains to be resolved. It has been reported that testosterone has no effect on GHR, whereas estradiol increases the alternatively spliced variant of the GHR gene, GH1 mRNA, but not the GHR mRNA (15). In contrast, administration of exogenous estradiol to immature rabbits reduces the liver GHR mRNA levels (16), and the nonaromatizable androgen DHT lowers GH-binding protein in pubertal humans (16).
Studies performed in experimental animals directed at clarifying sex-based differences in neuroendocrine regulation of the somatotropic axis suggest that sex differences in pulsatile GH secretion could originate from a reduced somatostatinergic inhibitory activity with or without increased GHRH activity (10, 11). Of special interest for understanding this mechanism is the study performed in our laboratory in fetal hypothalamic cells in primary culture, in which estrogens inhibited SS release with no modification of SS synthesis or GHRH secretion (17).
Consequently, at present, the mechanisms by which androgens regulate the somatotropic axis are not completely understood. Most of the studies have been performed in adult gonadectomized male rats in which either some androgenic effect remained, corresponding to a low-androgen, low-estrogen model (18), or nonandrogenic testicular regulators of somatotropic axis are removed (13, 19, 20). In the present study, to assess the relative role of androgens and estrogens on the regulation of somatotropic axis and somatic growth during pubertal development, we have used the androgen receptor-blocking factor flutamide, which is devoid of androgen effect and produces a low-androgenic, high-estrogenic biologic situation. Flutamide was given to 30-d-old normal male rats for 4 wk, a period in which almost the whole puberty is included (21).
METHODS
Animals and experimental design.
Thirty-day-old male Wistar rats, raised in our animal facilities, weighing about 100 g, were used. The maintenance and handling of the animals were performed as recommended by the National Institutes of Health guidelines on the care and use of laboratory animals according to the principles expressed in the Declaration of Helsinki and approved by the Institutional Review Board. Animals were housed in a constant 12-h light, 12-h dark cycle in a humidity-controlled room with a temperature of 22 ± 1°C. They were given free access to rat chow and tap water.
Flutamide (4-nitro-3-trifluro-methyl-isobutyranilide; Lassa, Barcelona, Spain) was diluted in 70% ethanol, and 100 mg/kg of body weight was subcutaneously. administered to each rat (n = 8). The control group (n = 8) was treated with the vehicle, 70% ethanol. This dose of flutamide has been demonstrated to completely inhibit the action of androgens (22). Animals were treated for 4 wk starting at postnatal day 30. This period covers the onset of pubertal development (which begins at postnatal day 34) and the whole pubertal period (which ends around postnatal day 60).
Blood collection and tissue removal.
The animals were killed by decapitation. Blood samples were collected from cervical vessels, allowed to clot at 4°C, and centrifuged at 1,500 rpm and 4°C for 15 min. Sera were separated and kept at −20°C until assayed for IR-GH that was measured with the kits provided by the National Hormone and Pituitary Program/National Institutes of Health (Bethesda, MD, U.S.A.). IGF-I was separated from its binding proteins by acid extraction and reverse-phase column chromatography procedure, using C18 Sep-Pack cartridges (Waters Millipore, Milford, MA, U.S.A.) and assayed by commercial IGF-I RIA kit (Nichols, San Juan Capistrano, CA, U.S.A.).
Pituitaries and brains were quickly removed and frozen in dry ice. Anterior pituitaries were separated from the neurohypophysis. The brain was dissected from the hypothalamus, as previously described (17). Pituitaries and hypothalami were divided into two halves for peptide extraction and RNA isolation.
Peptide extraction from pituitaries and hypothalami.
Peptide extraction was performed as previously described (17). Briefly, pituitaries and hypothalami were homogenized on ice by sonication in 1 M acetic acid, followed by boiling for 5 min and centrifugation (17,000 rpm; 30 min; 4°C). After adsorption on C18 Sep-Pack cartridges (Waters Associates), supernatants were eluted, lyophilized, reconstituted in 0.01 M HCl, and stored at −20°C until assayed for IR-SS, GHRH, and GH content. Protein recovery was between 90 and 100%, as previously described (17). Before purification, aliquots from supernatants were taken and used to determine total proteins by the Bradford method. All samples from the same experiment were extracted on the same day under identical conditions and assayed in the same RIA. The tissue peptide content was expressed as a function of the total protein.
IR-SS was quantified by a well-characterized RIA using the antiserum against SS-14 previously described (23). The final dilution of the antiserum was 1:300,000, and the cross-reactivity with SS-28 was 20%. The assay sensitivity was 1 pg/tube. The intra- and interassay variations were 8% and 15%, respectively. IR-GHRH was quantified by a well-characterized RIA using the antiserum against rat GHRH (1–43) previously described (24). The final dilution of the antiserum was 1:15,000. The assay sensitivity was 7.8 pg/tube. The intra- and interassay variations were 4.3% and 8.7%, respectively.
RNA extraction and analysis of GH and SS mRNA.
Total RNA was extracted by the method described by Chomczynski and Sacchi (25). Northern blot analysis of GH and SS mRNAs was performed as previously described (26). For GH mRNA analysis, a 32P-labeled Hin dIII fragment of the plasmid p-rGH1 was used as a probe by random primer extension (Boehringer Mannheim, Indianapolis, IN, U.S.A.) according to the protocol provided by the company. SS cDNA in the pSP65 vector (Promega Corp., Madison, WI, U.S.A.) (27) was used to produce a 32P-labeled antisense RNA probe after Sal I linearization and SP6 RNA polymerase synthesis. The autoradiograms shown are from a single experiment that is representative of two or three separate experiments. Equal loading was confirmed by comparing intensities of ethidium bromide-stained ribosomal 28S RNA in the nylon filter. The intensities of bromide-stained filters and autoradiogram signal levels were quantified by densitometry. Data were expressed as arbitrary units after correction for the 28S ribosomal band.
For IGF-I and GHR mRNA analysis, the RPA was used. A rat IGF-I cDNA (28) probe inserted in the pGEM-3 vector (Promega) was linearized with Hin dIII. An antisense RNA was constructed using [α-32P]UTP and T7 RNA polymerase. This probe was designed to allow the simultaneous detection of mRNAs containing or lacking the 52-bp insert in the E domain of some, but not all, rat IGF-I mRNA, which is presumably caused by alternative splicing of the primary IGF-I transcript (28). The RPA showed two major protected fragments of 376 bp (IGF-Ib) and 224 bp (IGF-Ia). The GHR cDNA (29) probe inserted in the pT2T3 18U vector (Pharmacia, Piscataway, NJ, U.S.A.) was linearized with Bam HI. An antisense RNA probe was generated using [α32P]UTP and T7 RNA polymerase. The RPA showed a major protected fragment of 450 bp. The cyclophilin cDNA (used as a loading control) inserted in pGEM-5Zf vector (Promega) was linearized with Apa I. An antisense RNA probe was generated using SP6 RNA polymerase and [α-32P]UTP. The RPA showed a protected fragment of 130 bp. Identical amounts of total RNA (15–20 μg) from each experimental group were hybridized at 45°C for 14–18 h. The hybridization solution contained 75% formamide, 80 mM Tris-HCl (pH 7.6), 4 mM EDTA, 1.6 M NaCl, 0.4% SDS, and the corresponding probe (5 × 105 cpm/reaction). After the hybridization, samples were digested using RNase A (40 μg/mL) and RNasa T1 (2 μg/mL), extracted with phenol-chloroform, and precipitated with ethanol. Then, samples were electrophoresed on an 8% polyacrylamide–8-M urea gel. Autoradiograms were developed after exposure to x-ray film (Kodak X-Omat, Rochester, NY, U.S.A.) at −80°C using two intensifying screens. Variations of gel loading were corrected against the corresponding cyclophilin blot values.
Quantification was performed by densitometric scanning using Adobe Photoshop 2.0 (Adobe Systems, Inc., Mountain View, CA, U.S.A.) and National Institutes of Health Image 1.47. The data represent the mean ± SEM.
Statistical analysis.
The quantifications of mRNA and peptide content in each tissue were analyzed three times. All data are expressed as the mean ± SEM. Statistical analysis was performed using t test. A p < 0.05 was considered significant.
RESULTS
Effect of androgen receptor blockade on gonadal steroid levels andsomatic growth.
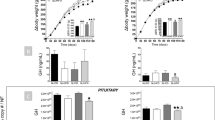
To study the mechanisms by which androgens modify the somatotropic axis and alter body growth, we used the nonsteroid-blocking androgen receptor factor, flutamide. The administration of flutamide to pubertal male rats for 4 wk not only markedly increased serum total testosterone levels (p < 0.001;Fig. 1A), but also significantly increased serum estradiol (p < 0.05;Fig. 1B), which reflects both peripheral aromatization of testosterone and testicular estrogen production. This finding demonstrates that the effects of flutamide administration reflect the blockade of androgen receptors as well as the unopposed high-estrogenic effect.
Effect of flutamide treatment on gonadal steroids and body weight in pubertal normal male Wistar rats. Flutamide was subcutaneously administered to 30-d-old male rats for 4 wk. A, serum total testosterone concentration. B, serum estradiol concentration. C, body weight gain. The values represent the mean ± SEM. *p < 0.05; **p < 0.01.
Body weight gain was decreased by chronic flutamide administration to pubertal male rats (Fig. 1C). There were no significant differences in daily food consumption between control and flutamide-treated animals (data not shown). These data indicate that alterations of the androgen to estrogen balance activity modify somatic growth during pubertal development in the feminized male phenotype rat.
Effect of androgen receptor blockade on hepatic IGF-I geneexpression and serum IGF-I.
As IGF-I has been shown to promote somatic growth and sexual development during puberty, we assessed the low-androgenic, high-estrogenic effect on hepatic and circulating IGF-I regulation. As shown in Figure 2, A and B, liver IGF-I mRNA accumulation was significantly reduced (p < 0.01) in the flutamide treated group of rats. This reduction of hepatic IGF-I gene expression coincided with a significant decrease (p < 0.05) of circulating IR-IGF-I in this group of relative high-estrogenic activity (Fig. 2C), indicating the mediation of hepatic IGF-I on the decreased somatic growth that occurs by the blockade of androgen receptor that produces a feminized male phenotype animal.
Effect of flutamide treatment on hepatic IGF-I in pubertal normal male Wistar rats. A, representative autoradiogram of an RPA of hepatic IGF-I. One major protected fragment of IGF-I mRNA can be detected, IGF-Ia (224 bp), and cyclophilin (102 bp) was used as an internal loading control. Lane 1, undigested IGF-I probe. Lane 2, IGF-I and cyclophilin probes after RNase A and T1 digestion. Lane 3, control group. Lane 4, flutamide-treated group. B, densitometric values of IGF-Ia mRNA fragments. Data are expressed as arbitrary units after correction for cyclophilin band (loading control). Each point represents the mean ± SEM of three determinations of IGF-I mRNA levels. Liver RNA was pooled from four different animals. C, serum IR-IGF-I levels. The values represent the mean ± SEM. *p < 0.05; **p < 0.01.
Action of flutamide administration on hepatic GHR geneexpression.
Liver IGF-I gene expression is a peripheral target of GH action, which is mediated by the interaction with its specific receptors. Therefore, to understand the mechanism of the IGF-I alterations, we have studied the GHR gene expression in the liver of the two groups of animals. The flutamide-treated rats showed a significant decrease of GHR mRNA levels (p < 0.01) as compared with the untreated group of animals (Fig. 3). This suggests that the reduction of IGF-I in the unbalanced high-estrogen group of animals is mediated by a decreased hepatic GHR gene expression and the consequent reduction of functional activity.
Effect of flutamide treatment on hepatic GHR in pubertal normal male Wistar rats. A, representative autoradiogram of an RPA of hepatic GHR. One major protected fragment of GHR mRNA can be detected (450 bp), and cyclophilin (102 bp) was used as an internal loading control. Lane 1, control group. Lane 2, flutamide-treated group. B, densitometric values of GHR mRNA fragments. Data are expressed as arbitrary units after correction for cyclophilin band (loading control). Each point represents the mean ± SEM of three determinations of GHR mRNA levels. Liver RNA was pooled from four different animals. The values represent the mean ± SEM. *p < 0.05; **p < 0.01.
Effect of androgen receptor blockade on pituitary GH geneexpression and GH secretion.
Moving upward to clarify other potential alterations of the somatotropic axis as a result of the modification of sex-steroid relative action, we have studied the pituitary GH gene expression and GH reserve and the serum random sample GH levels in these experimental conditions.
As shown in Figure 4, A and B, pituitary GH mRNA accumulation was not altered by the administration of flutamide. Surprisingly, pituitary IR-GH content was not different in the two groups (Fig. 4C), nor was there any difference in the random serum GH levels. These data indicated that the major action of a high unbalanced estrogen activity on the somatotropic axis occurs in the liver. No effect was shown at the pituitary level although this study does not exclude modifications in the mode of pulsatile GH secretion that must be accepted from other studies.
Effect of flutamide on pituitary GH regulation. A, pituitary IR-GH stores. B, serum IR-GH levels. C, pituitary GH mRNA content. The upper panel shows a representative autoradiogram of a Northern blot of pituitary GH. The lower panel shows the same filter with ethidium bromide-stained 28S ribosomal, which was used as an internal control. D, shows the densitometric values. GH mRNA and ethidium bromide-stained 28S were quantified by densitometry, and data are expressed as arbitrary units after correction for 28S ribosomal band. Each point represents the mean ± SEM of three determinations of GH mRNA levels. In this representative Northern blot, a pool of four half-pituitaries from different animals was used for each lane. E, pituitary IR-IGF-I stores. The values represent the mean ± SEM. *p < 0.05.
Additional data were obtained from the quantification of IR-IGF-I in pituitary extracts from these animals. As shown in Figure 4E, the flutamide-treated group had a significantly (p < 0.05) increased pituitary IR-IGF-I content compared with the untreated animals. This alteration might be involved in the local mechanism responsible for the unaltered pituitary GH gene expression and secretion in a situation of lower circulating IGF-I.
Effect of androgen receptor blockade on hypothalamic SS andGHRH.
Inasmuch as abundant sex-steroid receptors have been found in the hypothalamus and specific hypothalamic functional modifications have been involved in the sex difference of pulsatile GH secretion, the two major hypothalamic GH secretagogues, SS and GHRH, were studied in this situation of unbalanced estrogen to androgen action in feminized male phenotype pubertal rats.
As shown in Figure 5A, there was a significant increase of IR-SS content in the hypothalamic extracts of flutamide-treated rats. In contrast, a striking reduction of hypothalamic SS mRNA accumulation was seen in the same group of animals (p < 0.01;Fig. 5, C and D). No significant modification of hypothalamic IR-GHRH was shown under the effect of flutamide administration (Fig 5B).
Effect of flutamide on hypothalamic SS and GHRH regulation. A, hypothalamic IR-SS stores. B, hypothalamic IR-GHRH stores. C, hypothalamic SS mRNA content. The upper panel shows a representative autoradiogram of a Northern blot of hypothalamic SS. The lower panel shows the same filter with ethidium bromide-stained 28S ribosomal, which was used as an internal control. D, shows the densitometric values. SS mRNA and ethidium bromide-stained 28S were quantified by densitometry, and data are expressed as arbitrary units after correction for 28S ribosomal band. Each point represents the mean ± SEM of three determinations of SS mRNA levels. In this representative Northern blot, a pool of four half-hypothalami from different animals was used for each lane. The values represent the mean ± SEM. *p < 0.05; **p < 0.01.
These data indicate that the situation of unopposed estrogen action reduces SS release and, in a direct or indirect manner, SS gene expression, and therefore this might be another critical level for the interaction between sex steroids and the somatotropic axis.
DISCUSSION
In the present study, the exposure to flutamide at the dose of 100 mg/kg/d, which has been shown to completely inhibit androgen actions (22, 30), for 4 wk during pubertal development resulted in the increase of serum testosterone and estradiol levels as expected. Previous studies have shown that the antiandrogenic effect of flutamide would block the inhibitory feedback of testosterone on LH secretion, thus resulting in a profound increase in LH production and serum testosterone concentration (31). The increase in serum estradiol observed in this experimental model might be caused by aromatization of testosterone both in the periphery and in the stimulated testis under the increased LH. Because flutamide has been shown to alter cytochrome P-450, we cannot discard the possibility that some of the alterations in sex-steroid synthesis and metabolism observed in this study are because of the modifications of P-450 (32, 33).
In this model of unbalanced estrogen to androgen action by flutamide-induced androgen resistance in a male phenotype male rat, somatic growth was decreased, confirming earlier reports (34, 35). The effect of flutamide on body weight gain observed in this study probably reflects alterations in somatic growth rather than changes in body composition, as previous studies have shown that in the pubertal (36) and testicular feminized (34) male rat, changes in body weight gain under the influence of sex steroids are accompanied by parallel changes in skeletal growth, although a more-complex difference in body composition cannot be discarded. This effect on somatic growth was coincident with a decrease in serum IGF-I and hepatic IGF-I mRNA content. Therefore, the effect of flutamide on body weight gain was probably mediated in part through the alteration of circulating IGF-I levels observed in the present study. In fact, exogenous administration of IGF-I has been shown to result in body growth, which demonstrates that IGF-I exerts a growth-promoting effect through endocrine mechanisms (37, 38). Furthermore, it has been shown that androgen-mediated effects on body weight gain are parallel to modifications in circulating IGF-I levels (2).
The primary source of circulating IGF-I has been demonstrated to be the liver. Our study shows that the long-term androgen receptor blockade induced by flutamide in pubertal male rats resulted in lower hepatic IGF-I mRNA levels. It is likely, therefore, that the low circulating IGF-I levels were caused by a decrease in hepatic IGF-I production. However, we cannot determine whether stability or transcriptional rate of hepatic IGF-I mRNA were altered by flutamide administration.
Additionally, our study shows that flutamide administration decreased hepatic GHR mRNA content, indicating a decrease of GHR gene expression. Thus, the unbalance of estrogen to androgen action produced by the flutamide-induced blockade of the androgen receptor appears to decrease hepatic GHR gene expression and consequently decrease the GH effectiveness in the liver, with lower IGF-I gene expression and IGF-I secretion in male pubertal rats. Our observation is supported by earlier reports in which long-term exposure of immature rabbits to estrogens resulted in a decrease in GHR mRNA levels (16). Also, and in agreement with our findings, estrogens have been shown to decrease circulating IGF-I and its hepatic gene expression (2), whereas androgens have the opposite effect on IGF-I regulation (39). Further confirmation of this mechanism was obtained from studies performed in our laboratory in which a diminished GHR gene expression was shown in the liver of pregnant rats, a model of physiologic hyperestrogenic activity (40). In contrast, other authors have shown that exposure of gonadectomized male rats whose endogenous testosterone was removed by castration to estrogens either did not alter liver GHR mRNA levels (41) or increased the alternatively spliced variant GH1 of the hepatic GHR mRNA (15). There are several explanations for these discrepancies. First of all, the effect of sex steroids on GH receptor gene expression is modulated by the levels of estrogens (40). The biphasic effect of estradiol on GH biologic actions has been well documented in humans and in rats (42). Additionally, the differences that exist in GH secretion rate and the mode of GH pulsatile secretion between adulthood and pubertal development and therefore the development stage at which our study was performed may explain some of these discrepancies. Alternatively, the remaining androgenic effect after male rat gonadectomy, the procedure by which androgen effect was altered in the previous studies, may be important for the regulatory effect on GHR gene expression (18). At least part of the decrease in hepatic IGF-I mRNA may account for the liver GH resistance observed in this study. However, sex steroids may also exert direct actions on IGF-I regulation.
Apart from the effect on target tissue responsiveness to GH, sex steroids can also influence somatic growth through modifications of GH secretion rate or the mode of its pulsatile secretion (43). Although spontaneous pulsatile GH secretion was not analyzed in this study, the lack of alteration in serum GH concentration, GH pituitary reserve, and its pituitary mRNA levels suggests that blockade of the androgen receptor by flutamide did not significantly alter GH secretion rate, but some influence on the amplitude or, less probably, the frequency of the GH secretory pulses can occur. Regarding the direct action of estradiol on the GH secretion and gene expression in the pituitary, not much effect may be expected. Only approximately 2.3% of GH-secreting cells in the normal rat express the estrogen receptor α-isoform (44). The limited information so far available indicates a higher coexpression of the estrogen receptor β-isoform in the GH-secreting cells of normal rat pituitary, but the functional implication of this isoform on GH gene expression and secretion is not well established.
In this long-term model, the major hypothalamic regulators of GH secretion were also studied to determine to what extent sex steroids regulate SS and GHRH in pubertal male rats and consequently influence GH secretion. We found that the blockade of androgen activity by flutamide reduced hypothalamic SS mRNA levels. Stimulation of hypothalamic SS gene expression by androgens through activation of its receptor has been reported (45), but it has not been well documented at puberty. Our data confirm that in the biologic situation of low androgen and high estradiol activity in male phenotype rats, SS gene expression is reduced, but these results do not allow us to assess whether this action is caused solely by the absence of androgens. We found that flutamide administration increased hypothalamic IR-SS content coincident with the reduction of SS mRNA, which suggests a decrease in SS release. We and others have shown that estrogens decreased SS release in fetal hypothalamic cells in primary culture (17). Therefore, it appears that the increment in estradiol observed in this study may in part contribute to the increase in hypothalamic SS content. We did not find any change in hypothalamic GHRH content. Although previous studies have shown that androgens increase hypothalamic GHRH gene expression (46), no data of sex-steroid effect on its peptide content have been reported. At the hypothalamic level, abundant expression of the estrogen receptor gene has been documented (47), and direct action of estradiol on SS and GHRH secretion has been demonstrated at least in fetal hypothalamic cells in primary culture (17). Therefore, the alteration of SS that occurs in this high-estradiol activity model of flutamide-treated rats might be mediated by the direct action of estradiol at the hypothalamic level. Whether these animals were converted from a male to a female pulsatile GH secretory pattern under this effect was not addressed in this study.
It is well known that IGF-I exerts a negative feedback effect on GH release and GH gene expression (48, 49), although it has not been well differentiated whether this effect is the result of the endocrine action of circulating IGF-I or is mediated by locally produced IGF-I acting in a paracrine or autocrine manner or both. In this study, we observed that flutamide administration increased pituitary IGF-I content, which could counteract the feedback effect of decreased serum IGF-I levels (50). The apparent discrepancy of the effect of flutamide treatment on liver IGF-I and on pituitary IGF-I may be attributed to the unbalanced increment of estradiol that occurs in this experimental situation. In fact, it has been reported that exposure to estradiol increased pituitary IGF-I gene expression (51), suggesting that the regulation of IGF-I by estradiol might be tissue specific.
In conclusion, this study demonstrates that the alteration of the physiologic balance of androgens to estrogens alters the normal body growth and suggests that this effect is partly mediated through decreased serum IGF-I levels. Also, the blockade of androgen receptors and the unopposed estrogenic action decrease hepatic IGF-I gene expression, the main source of circulating IGF-I, with no modification of pituitary GH gene expression or GH reserve. The decrease in GHR gene expression by flutamide administration may account for a lower hepatic responsiveness to GH, which is believed to be the main regulator of the liver IGF-I, and may justify the negative action of the androgen activity blockade with unbalanced estrogenic effect on hepatic IGF-I gene expression. We confirm that in this low-androgen and high-estradiol activity model, at pubertal development, hypothalamic SS gene expression was reduced at the same time as an increase in IR-SS occurred, whereas there was no modification in hypothalamic IR-GHRH content. The absence of changes in random serum GH samples and pituitary GH production probably reflects the adjustment to the feedback effect of both circulating and pituitary IGF-I and to the changes in the hypothalamic regulators.
This study also indicates that, during pubertal development, the mechanism of sex difference in somatic growth may be a decreased GH effectiveness caused by the negative action of relative high-estrogen effect on the hepatic GHR gene expression and IGF-I gene expression. Whether this is a direct estrogenic effect on the liver GHR gene or is mediated through modifications of pulsatile GH secretion or GH secretion rate is not addressed in this study.
Abbreviations
- DHT:
-
dihydrotestosterone
- GHR:
-
GH receptor
- GHRH:
-
GH-releasing hormone
- IR-GH:
-
immunoreactive GH
- IR-GHRH:
-
immunoreactive GHRH
- IR-SS:
-
immunoreactive somatostatin
- RPA:
-
ribonuclease protection assay
- SS:
-
somatostatin
References
Sandstedt J, Ohlsson C, Norjavaara E, Nilsson J, Tornell J 1994 Disproportional bone growth and reduced weight gain in gonadectomized male bovine growth hormone transgenic and normal mice. Endocrinology 135: 2574–2580
Borski RJ, Tsai W, DeMott-Friberg R, Barkan AL 1996 Regulation of somatic growth and the somatotropic axis by gonadal steroids: primary effect on insulin-like growth factor I gene expression and secretion. Endocrinology 137: 3253–3259
Gabriel SM, Roncancio JR, Ruiz NS 1992 Growth hormone pulsatility and the endocrine milieu during sexual maturation in male and female rats. Neuroendocrinology 56: 619–625
Wehrenberg WB, Giustina A 1992 Basic counterpoint: mechanisms and pathways of gonadal steroid modulation of growth hormone secretion. Endocr Rev 13: 299–308
Jansson JO, Ekberg S, Isaksson OG, Eden S 1984 Influence of gonadal steroids on age- and sex-related secretory patterns of growth hormone in the rat. Endocrinology 114: 1287–1294
Hasegawa O, Sugihara H, Minami S, Wakabayashi I 1992 Masculinization of growth hormone (GH) secretory pattern by dihydrotestosterone is associated with augmentation of hypothalamic somatostatin and GH-releasing hormone mRNA levels in ovariectomized adult rats. Peptides 13: 475–481
Schwander JC, Hauri C, Zapf J, Froesch ER 1983 Synthesis and secretion of insulin-like growth factor and its binding protein by the perfused rat liver: dependence on growth hormone status. Endocrinology 113: 297–305
Phillip M, Palese T, Hernandez ER, Roberts CT Jr, LeRoith D, Kowarski AA 1992 Effect of testosterone on insulin-like growth factor-I (IGF-I) and IGF- I receptor gene expression in the hypophysectomized rat. Endocrinology 130: 2865–2870
Metzger DL, Kerrigan JR 1993 Androgen receptor blockade with flutamide enhances growth hormone secretion in late pubertal males: evidence for independent actions of estrogen and androgen. J Clin Endocrinol Metab 76: 1147–1152
Veldhuis JD 1996 Gender differences in secretory activity of the human somatotropic (growth hormone) axis. Eur J Endocrinol 134: 287–295
Tannenbaum GS 1993 Genesis of episodic growth hormone secretion. J Pediatr Endocrinol 6: 273–282
Mathews LS, Enberg B, Norstedt G 1989 Regulation of rat growth hormone receptor gene expression. J Biol Chem 264: 9905–9910
Gevers EF, Wit JM, Robinson IC 1995 Effect of gonadectomy on growth and GH responsiveness in dwarf rats. J Endocrinol 145: 69–79
Frick GP, Leonard JL, Goodman HM 1990 Effect of hypophysectomy on growth hormone receptor gene expression in rat tissues. Endocrinology 126: 3076–3082
Gabrielsson BG, Carmignac DF, Flavell DM, Robinson IC 1995 Steroid regulation of growth hormone (GH) receptor and GH-binding protein messenger ribonucleic acids in the rat. Endocrinology 136: 209–217
Domene HM, Marin G, Sztein J, Yu YM, Baron J, Cassorla FG 1994 Estradiol inhibits growth hormone receptor gene expression in rabbit liver. Mol Cell Endocrinol 103: 81–87
Fernández G, Sánchez-Franco F, de los Frailes MT, Tolón RM, Lorenzo MJ, López J, Cacicedo L 1992 Regulation of somatostatin and growth hormone-releasing factor by gonadal steroids in fetal rat hypothalamic cells in culture. Regul Pept 42: 135–144
Marchetti B, Poulin R, Plante M, Labrie F 1988 Castration levels of plasma testosterone have potent stimulatory effects on androgen-sensitive parameters in the rat prostate. J Steroid Biochem 31: 411–419
Lago F, Señarís RM, Emson PC, Dominguez F, Dieguez C 1996 Evidence for the involvement of non-androgenic testicular factors in the regulation of hypothalamic somatostatin and GHRH mRNA levels. Brain Res Mol Brain Res 35: 220–226
Aguilar E, Pinilla L, Tena-Sempere M 1993 Growth hormone-releasing hormone-induced growth hormone secretion in adult rats orchidectomized or injected with ethylene dimethane sulphonate. Neuroendocrinology 57: 132–134
Ojeda SR, Andrews WW, Advis JP, White SS 1980 Recent advances in the endocrinology of puberty. Endocr Rev 1: 228–257
Imperato-McGinley J, Sanchez RS, Spencer JR, Yee B, Vaughan ED 1992 Comparison of the effects of the 5 alpha-reductase inhibitor finasteride and the antiandrogen flutamide on prostate and genital differentiation: dose-response studies. Endocrinology 131: 1149–1156
de los Frailes MT, Cacicedo L, Lorenzo MJ, Fernández G, Sánchez-Franco F 1988 Thyroid hormone action on biosynthesis of somatostatin by fetal rat brain cells in culture. Endocrinology 123: 898–904
Fernández G, Cacicedo L, Lorenzo MJ, de los Frailes MT, Sánchez Franco F 1989 Growth hormone-releasing factor production by fetal rat cerebrocortical and hypothalamic cells in primary culture. Endocrinology 125: 1991–1998
Chomczynski P, Sacchi N 1987 Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–159
López-Fernández J, Sánchez-Franco F, Velasco B, Tolón RM, Pazos F, Cacicedo L 1996 Growth hormone induces somatostatin and insulin-like growth factor I gene expression in the cerebral hemispheres of aging rats. Endocrinology 137: 4384–4391
Tolón RM, Sánchez-Franco F, de los Frailes MT, Lorenzo MJ, Cacicedo L 1994 Effect of potassium-induced depolarization on somatostatin gene expression in cultured fetal rat cerebrocortical cells. J Neurosci 14: 1053–1059
Lowe WL Jr, Lasky SR, LeRoith D, Roberts CT Jr 1988 Distribution and regulation of rat insulin-like growth factor I messenger ribonucleic acids encoding alternative carboxyterminal E- peptides: evidence for differential processing and regulation in liver. Mol Endocrinol 2: 528–535
Bornfeldt KE, Arnqvist HJ, Enberg B, Mathews LS, Norstedt G 1989 Regulation of insulin-like growth factor-I and growth hormone receptor gene expression by diabetes and nutritional state in rat tissues. J Endocrinol 122: 651–656
Tena-Sempere M, Pinilla L, Aguilar E 1993 Follicle-stimulating hormone and luteinizing hormone secretion in male rats orchidectomized or injected with ethylene dimethane sulfonate. Endocrinology 133: 1173–1181
Marchetti B, Labrie F 1988 Characteristics of flutamide action on prostatic and testicular functions in the rat. J Steroid Biochem 29: 691–698
Brandes LJ, Queen GM, LaBella FS 1997 Salutary clinical response of prostate cancer to antiandrogen withdrawal: assessment of flutamide in an in vitro paradigm predictive of tumor growth enhancement. Clin Cancer Res 3: 1357–1361
Reznikov A, Korpacheva T 1990 Nonsteroid antiandrogen inhibiting effect on testosterone metabolism in rat prostate and liver. Endocrinol Exp 24: 437–447
Vanderschueren D, Van Herck E, Geusens P, Suiker A, Visser W, Chung K, Bouillon R 1994 Androgen resistance and deficiency have different effects on the growing skeleton of the rat. Calcif Tissue Int 55: 198–203
Vanderschueren D, Van Herck E, Suiker AM, Visser WJ, Schot LP, Chung K, Lucas RS, Einhorn TA, Bouillon R 1993 Bone and mineral metabolism in the androgen-resistant (testicular feminized) male rat. J Bone Miner Res 8: 801–809
Jansson JO, Eden S, Isaksson O 1983 Sites of action of testosterone and estradiol on longitudinal bone growth. Am J Physiol 244: E135–E140
van Buul-Offers S, Ueda I, Van den Brande JL 1986 Biosynthetic somatomedin C (SM-C/IGF-I) increases the length and weight of Snell dwarf mice. Pediatr Res 20: 825–827
Hizuka N, Takano K, Shizume K, Asakawa K, Miyakawa M, Tanaka I, Horikawa R 1986 Insulin-like growth factor I stimulates growth in normal growing rats. Eur J Pharmacol 125: 143–146
Crawford BA, Singh J, Simpson JM, Handelsman DJ 1993 Androgen regulation of circulating insulin-like growth factor-I during puberty in male hypogonadal mice. J Endocrinol 139: 57–65
Escalada J, Sánchez-Franco F, Velasco B, Cacicedo L 1997 Regulation of growth hormone (GH) gene expression and secretion during pregnancy and lactation in the rat: role of insulin-like growth factor- I, somatostatin, and GH-releasing hormone. Endocrinology 138: 3435–3443
Baumbach WR, Bingham B 1995 One class of growth hormone (GH) receptor and binding protein messenger ribonucleic acid in rat liver, GHR1, is sexually dimorphic and regulated by GH. Endocrinology 136: 749–760
O'Sullivan AJ, Crampton LJ, Freund J, Ho KK 1998 The route of estrogen replacement therapy confers divergent effects on substrate oxidation and body composition in postmenopausal women. J Clin Invest 102: 1035–1040
Kerrigan JR, Rogol AD 1992 The impact of gonadal steroid hormone action on growth hormone secretion during childhood and adolescence. Endocr Rev 13: 281–298
Friend KE, Chiou YK, Lopes MB, Laws ER Jr, Hughes KM, Shupnik MA 1994 Estrogen receptor expression in human pituitary: correlation with immunohistochemistry in normal tissue, and immunohistochemistry and morphology in macroadenomas. J Clin Endocrinol Metab 78: 1497–1504
Argente J, Chowen-Breed JA, Steiner RA, Clifton DK 1990 Somatostatin messenger RNA in hypothalamic neurons is increased by testosterone through activation of androgen receptors and not by aromatization to estradiol. Neuroendocrinology 52: 342–349
Zeitler P, Argente J, Chowen-Breed JA, Clifton DK, Steiner RA 1990 Growth hormone-releasing hormone messenger ribonucleic acid in the hypothalamus of the adult male rat is increased by testosterone. Endocrinology 127: 1362–1368
Shughrue PJ, Lane MV, Merchenthaler I 1997 Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol 388: 507–525
Yamashita S, Melmed S 1986 Insulin-like growth factor I action on rat anterior pituitary cells: suppression of growth hormone secretion and messenger ribonucleic acid levels. Endocrinology 118: 176–182
Namba H, Morita S, Melmed S 1989 Insulin-like growth factor-I action on growth hormone secretion and messenger ribonucleic acid levels: interaction with somatostatin. Endocrinology 124: 1794–1799
Fagin JA, Brown A, Melmed S 1988 Regulation of pituitary insulin-like growth factor-I messenger ribonucleic acid levels in rats harboring somatomammotropic tumors: implications for growth hormone autoregulation. Endocrinology 122: 2204–2210
Michels KM, Lee WH, Seltzer A, Saavedra JM, Bondy CA 1993 Up-regulation of pituitary [125I]insulin-like growth factor I (IGF-I) binding and IGF binding protein-2 and IGF-I gene expression by estrogen. Endocrinology 132: 23–29
Acknowledgements
The authors thank Mary Harper for the preparation of the manuscript, the National Hormone and Pituitary Program NIDDK for supplying the rat GH RIA reagents, and Maria Paz Muñoz and Costanza Navarro for technical assistance.
Author information
Authors and Affiliations
Additional information
Supported by a Beca de Ampliación de Estudios FIS (94/5062), and grants from FIS (99/204 and 94/0308) and from the Ministerio de Educación y Ciencia (PB 95/0258 and PB94/1271).
Rights and permissions
About this article
Cite this article
Pazos, F., Sánchez-Franco, F., Balsa, J. et al. Mechanisms of Reduced Body Growth in the Pubertal Feminized Male Rat: Unbalanced Estrogen and Androgen Action on the Somatotropic Axis. Pediatr Res 48, 96–103 (2000). https://doi.org/10.1203/00006450-200007000-00017
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-200007000-00017
This article is cited by
-
Hormonal Correlates of Divergent Growth Trajectories in Wild Male Anubis (Papio anubis) and Hamadryas (P. hamadryas) Baboons in the Awash River Valley, Ethiopia
International Journal of Primatology (2013)