Abstract
In late gestation, Ca2+ transport across the human placenta must increase in response to the demands of accelerating bone mineralization of the fetus. This is an ATP-dependent transport against a concentration gradient across the basal or the fetal-facing plasma membrane of the syncytiotrophoblast. The aims of the present study were to determine the relationship between ATP-dependent Ca2+ transport and gestational age in the third trimester and to identify the specific isoforms of plasma membrane Ca2+ ATPase (PMCA) present in human syncytiotrophoblast. Basal membrane vesicles were isolated from normal placentas and from placentas obtained from preterm deliveries with no other complications (32–37 wk of gestation). We studied the uptake of 45Ca2+ into basal membrane vesicles in the absence and presence of ATP by using rapid filtration techniques. Western blot was used to assess the protein expression of the PMCA isoforms 1–4. Isoforms 1 and 4 of PMCA were identified in basal membrane of human placenta. The ATP-dependent Ca2+ transport increased linearly during the third trimester (r = 0.571, p = 0.0015, n = 28). However, PMCA protein expression was unaltered during the same period of gestation. Our results show that PMCA in the fetal-facing plasma membrane of the human syncytiotrophoblast is markedly activated toward the end of pregnancy. We suggest that these changes are critical in supplying the rapidly growing fetus with sufficient Ca2+ for bone mineralization.
Similar content being viewed by others
Main
The fetus is hypercalcemic relative to the mother, and the final step in the maternal-fetal transfer of Ca2+ involves transport against a concentration gradient across the BM or fetal-facing plasma membrane of the syncytiotrophoblast. Several studies have demonstrated an ATP-dependent transport activity in BM vesicles (1, 2). This transport is suggested to be performed by the PMCA, which mediates the extrusion of Ca2+ from the cell interior. Immunohistochemistry has shown specific staining of PMCA in the BM of human placenta (3), the distal convoluted tubules of human kidney (4), and rat small intestine (5). Kikuchi et al. (6) have shown an ATP-dependent Ca2+ transport across basolateral membrane vesicles of human small intestine. The influx of calcium into the syncytiotrophoblast cell across the MVM of human placenta has been suggested to be performed by a facilitated diffusion process (7), whereas the transport across the enterocyte brush membrane involves opening of voltage-gated calcium channels (8). Influx into the syncytiotrophoblast and enterocyte has been suggested to be aided by calcium-binding proteins (9). In recent studies, the calcium-binding protein calbindin 9k has been found at the mRNA level in both rat and human placenta (10, 11).
Four isoforms of PMCA have been identified in human tissues (12). Two isoforms of PMCA, 1 and 4, appear to be expressed at the mRNA level in the human placenta (13). Although the PMCA protein has been shown to be present in BM (3, 14), the specific isoform expression remains to be established. The distribution of the PMCA isoforms in BM may play a physiologic role because they are suggested to have different mechanisms of activation (15, 16).
In late gestation, Ca2+ transport across the placenta must increase in response to the demands of accelerating bone mineralization of the fetus (17, 18). At the end of the second trimester (wk 24), the human fetal skeleton accumulates 60 mg of Ca2+ each day. At the time of birth (wk 40), Ca2+ accumulation increases to 340 mg/d (19, 20). In the rat, a 2–3-fold increase of the PMCA gene expression and a 72-fold rise in the calcium transport across the placenta occurs during the last days of gestation (10). In this study, a 135-fold increase in rat placental calbindin 9k was found, and the authors suggested that this protein is the rate-limiting step in rat placental calcium transport. The mRNA levels for calbindin 9k in the human placenta are fairly constant throughout gestation (11). The mechanisms responsible for the late gestational increase in transplacental Ca2+ transport in the human are unknown. However, it could be due to an increased protein expression, increased placenta size, and/or activation of the existing PMCA proteins.
The aims of the present study were to establish the isoform identity of PMCA in the BM and to study the protein expression and activity of this transporter throughout the third trimester of pregnancy in the human. We isolated BM vesicles from placentas obtained between 32–42 wk of gestation. PMCA isoforms and protein expression were characterized by means of Western blotting employing isoform-specific antibodies. In addition, ATP-dependent calcium uptake of 45Ca2+ was studied using rapid filtration techniques.
METHODS
Vesicle preparation.
Placentas were obtained from uncomplicated term pregnancies (38–42 wk) as well as from pregnancies of 32–37 wk of gestation with no complication other than prematurity. The collection of placental tissue was approved by the Committee for Research Ethics at Göteborg University. Tissue was processed within 30 min of vaginal or cesarean delivery. Syncytiotrophoblast BM vesicles were isolated by the method of Illsley et al. (21). The chorionic plate and decidua were removed, and approximately 100 g of villous tissue was cut into small pieces and then washed in buffered saline. The tissue was homogenized on ice in 250 mL of Buffer D (250 mM sucrose, 10 mM HEPES/Tris, 1.6 μM antipain, 0.7 μM pepstatin A, 0.5 μM aprotinin, 1 mM EDTA) at 15,000 rpm for 2 min using a polytron (Kinematica Ag, Switzerland), followed by centrifugation (10,000 ×g) at 4°C for 15 min. The supernatant was saved, and the pellet was rehomogenized in 100 mL of Buffer D, followed by centrifugation (10,000 ×g) at 4°C for 10 min. The second supernatant was added to the first, and the pellet was discarded. Magnesium chloride (12 mM) was added to the combined supernatant, which was stirred on ice for 20 min. The mixture was then centrifuged (2500 ×g) for 10 min. The pellet contains the BM and the supernatant MVM. The MVM fraction was centrifuged (125,000 ×g) for 30 min. The BM was further purified by sucrose-gradient centrifugation (21). The sucrose gradient was centrifuged (140,000 ×g) for 60 min at 4°C. BM were recovered from between the bottom (density 1.192 g/cm3) and middle sucrose layer (1.160 g/cm3), resuspended in Buffer D, and centrifuged (125,000 ×g) for 30 min at 4°C. MVM and BM vesicles were snap-frozen in liquid nitrogen and stored at −80°C.
The purity of the membrane preparation was assessed using standard activity assays for adenylate cyclase (22) and alkaline phosphatase (23). The production of cAMP by adenylate cyclase was measured by RIA (New England Nuclear, Boston, MA, U.S.A.). Further assays for contamination by endoplasmic reticulum [NADPH cytochrome c (24)] and mitochondria [succinate dehydrogenase (25)] were performed. The enrichment values were calculated as the ratio of the enzyme activity in the purified membrane preparation and the activity in the homogenate or postnuclear membrane fraction. All protein concentrations were determined according to the method of Bradford (26), using the Bio-Rad protein assay procedure (Bio-Rad, Hercules, CA, U.S.A.) and BSA as a standard.
Immunocytochemistry.
Tissue samples from full-term human placenta were rinsed in ice-cold physiologic saline and fixed in a solution of zinc salts, a modified method by Johansson et al. (27). The tissue was embedded in paraffin and then sectioned 4-μm thick. The sections were incubated overnight in primary antibody, monoclonal anti-PMCA ATPase antibody 5F10 (Affinity Bioreagents, Golden, CO, U.S.A.), at a 1:500 dilution in normal horse serum-blotto. Secondary biotinylated antibodies at a 1:500 dilution in PBS were used, and the slides were incubated for 60 min at room temperature. An avidin-peroxidase complex was layered on top of the secondary antibody (Vector Laboratories, Inc., Burlingame, CA, U.S.A.). The slides were then treated with diamino benzidine (DAB), followed by dehydrations in graded ethanol. In negative controls, the primary antibody was replaced by mouse ascites fluid.
Western blot.
Western blots were used for quantification of the 140-kD PMCA protein. Twenty micrograms of total BM protein was separated on a 7.5% SDS-PAGE gel and transferred to a Hybond enhanced chemi lumininescence (ECL) nitrocellulose membrane (Amersham, UK) at 30 V overnight using Bio-Rad Blot Cell (Bio-Rad, Hercules, CA, U.S.A.). After transfer, the membrane was blocked in PBS, 0.1% Tween 20, 5% nonfat milk for 60 min at room temperature. After 1 × 15 min and 2 × 5 min washes in PBS/0.1% Tween 20, the membrane was incubated for 60 min with primary antibody (5F10) at 1:1000 dilution. This antibody is directed toward all of the four known isoforms of the PMCA. The membrane was then washed in PBS/0.1% Tween 20, and bound antibody was detected using horseradish peroxidase-linked anti-mouse Ig (1:1000 dilution) and the ECL detection kit (Amersham, UK). Western blots were also used to identify the presence of specific isoforms of PMCA in BM. The isoform-specific antibodies were a generous gift from Prof. E. Carafoli, Institute of Biochemistry, Swiss Federal Institute of Technology. Each antibody is directed toward the N-terminal region of the protein, which differs substantially in the first 80–90 amino acids. The specificity of the antibodies was tested by determining cross-reactivity using proteins overexpressed in Sf9 cells (12). The Western blot protocol described above was used with small changes to determine the isoform expression in isolated placenta membranes. Briefly, rat brain homogenate (15 mg) was used as control, and 30 mg of BM was separated on 7.5% SDS PAGE gel. The incubation with both primary (1:500) and secondary (1:1000) antibodies was performed in 5% nonfat milk for 60 min at room temperature. All washing steps were performed as described above. Densitometry of the autoradiography for PMCA expression during gestation was performed using IP Lab gel (Signal Analytics Corporation, Vienna, VA, U.S.A.). Both the raw densitometry values and the relative density (determined by dividing the density of each sample with the mean density of all samples) were analyzed. No bands were observed in control experiments in which the primary antibody was omitted.
Calcium uptake.
The uptake of calcium by BM vesicles was measured by standard rapid filtration techniques according to the method of Fisher et al. (1). The reaction mixture contained 100–200 μg of total vesicle protein in 240 mM sucrose, 5 mM MgCl2, 0.2 mM EGTA, 0.2 mM CaCl2, 1 μCi 45CaCl2 (Amersham, UK), 10 mM HEPES/Tris, pH 7.4 at 37°C in a total volume of 0.5 mL. The incubation of the reaction mixture was allowed to proceed for the indicated times (5–60 min) at 37°C in the presence and absence of 5 mM ATP-MgCl2. In the inhibition study, vesicles were preincubated with inhibitor for 30 min before being added to the reaction mixture and then incubated for 10 min at 37°C. Calcium uptake was stopped by the addition of 5 mL ice-cold wash solution (10 mM HEPES/Tris, 250 mM sucrose, 4 mM EGTA, pH 7.4 at 4°C) and, subsequently, the reaction mixture was filtered under vacuum on 0.45-μm nitrocellulose filters (Millipore, Bedford, MA, U.S.A.) that had been prewashed with 10 mM HEPES/Tris, 250 mM sucrose, 5 mM CaCl2 (pH 7.4 at 4°C). The filter was washed three times with 2 mL of wash solution. The radioactivity retained on the filter was measured by liquid scintillation. The ATP-dependent calcium uptake was calculated as the difference between Ca2+ uptake in the presence and absence of ATP. Calcium uptake was expressed as nmol of Ca2+/mg total membrane protein.
Data analysis.
Statistical significance of differences between groups was determined using paired t test or repeated-measures ANOVA and Dunnett's test. Data are expressed as mean ± SEM unless otherwise specified. Regression analysis was carried out using Statview (Abacus Concepts, Berkley, CA, U.S.A.).
RESULTS
Purity of membrane preparations.
The enrichment in adenylate cyclase activity was 43-fold, whereas alkaline phosphatase activity was 4-fold in our BM preparation (Table 1). NADPH cytochrome c and succinate dehydrogenase were both low in the BM preparation (4- and 3-fold enrichment, respectively). We found no significant differences in the enrichment of BM isolated from preterm placentas (results not shown).
Immunocytochemistry.
Figure 1A shows the distribution of PMCA in human syncytiotrophoblast. The DAB reaction product was localized to the BM (indicated by arrowhead). Negative controls did not show significant nonspecific staining (Fig. 1B).
Immunocytochemistry performed on a section (4 μM) of paraffin-embedded human term placenta villous tissue. (A) Primary antibody (5F10), which recognizes all four PMCA isoforms. The arrow indicates the MVM, and the arrowhead indicates BM. (B) Control section in which primary antibody was replaced by ascites fluid. Magnification ×1000, bar = 10 μm.
Western blot analyses.
We have investigated the presence of the four different isoforms of the human PMCA in BM (Fig. 2). Both isoform 1 and 4 were present in the BM with a molecular mass of approximately 140 kD. In the rat brain homogenate, isoform 1 was present at the same molecular mass. Isoforms 2 and 3 were also present in the rat brain homogenate with molecular mass of 140 kD, but these isoforms were not present in the human placental membrane.
Isoforms of the PMCA in the human placental syncytiotrophoblast BM. Rat brain homogenate (R, 15 μg) and BM (30 μg) were separated by 7.5% SDS-PAGE. 1, Antibody for isoform 1N;2, antibody for isoform 2N;3, antibody for isoform 3N; and 4, antibody for isoform 4N. The BM were isolated from a 38-wk human placenta.
We used Western blot to investigate possible changes in PMCA expression in BM during the last trimester of pregnancy by using a monoclonal antibody that recognizes all four isoforms. Figure 3, shows a representative Western blot of the PMCA protein expression over gestation. In Figure 4 the relative density of PMCA expression over gestation for all samples is plotted. The Western blots were performed in two runs with random samples across the gestation, and there was no significant relationship between the PMCA expression and gestational age from 32 wk of gestation until term (n = 28).
Autoradiography of Western blot for PMCA expression over gestation. BM protein (20 μg) of the syncytiotrophoblast isolated from human placentas at different gestational ages was separated on 7.5% SDS-PAGE and then probed for PMCA expression by using an antibody directed against all four isoforms of the Ca2+ pump. Labels indicate gestational age for each sample in weeks.
ATP-dependent Ca2+ transport.
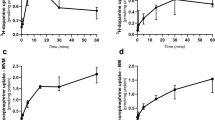
The time dependence of Ca2+ uptake into BM was studied at 37°C in the presence and absence of 5 mM ATP-MgCl2 (Fig. 5). The ATP-dependent Ca2+ transport was linear for the first 10 min of incubation. After 10 min, the rate of ATP-dependent Ca2+ accumulation gradually diminished. In the absence of ATP, there was no significant accumulation of calcium during the first 30 min of incubation.
Figure 6 shows the effect of various inhibitors on the ATP-dependent Ca2+ uptake. We have used a calcium-specific ionophore, A23187, to exclude the possibility that the ATP-dependent accumulation was due to calcium binding to the membrane. A23187 (10 μM) was added to the reaction mixture, which was subsequently incubated at 37°C for 30 min. The presence of the Ca2+ ionophore decreased ATP-dependent Ca2+ uptake by 91% (n = 5, p < 0.05). The Mg2+ dependence of the calcium uptake was tested by replacing the ATP-MgCl2 with Tris-ATP. In the absence of Mg2+, the ATP-dependent calcium uptake was decreased by 76% (n = 6, p < 0.05). To investigate the specificity of the Ca2+-ATPase activity, we used a variety of P-pump inhibitors, vanadate, strophanthidin, and LaCl3, at final concentrations of 100 μM (28–30). The ATP-dependent calcium uptake was decreased by 56, 63, and 86%, respectively (n = 6, p < 0.05).
Characterization of the ATP-dependent Ca2+ uptake into BM vesicles. The uptake in the presence of 5 mM MgCl2-ATP after 30 min of incubation at 37°C was considered to represent 100%. Addition of the calcium ionophore 10 μM A23187 and removal of Mg2+ resulted in nearly complete inhibition of the Ca2+ uptake. The ATP-dependent Ca2+ uptake was inhibited to varying degrees by 100 μM vanadate, strophanthidin, or LaCl3 (56, 63, and 86%, respectively; ★p < 0.05 vs MgCl2-ATP;n = 5–6).
We measured calcium uptake into BM after 10 min of incubation at 37°C from samples taken over the last trimester of gestation. We found a significant linear relationship (y = −168 + 5.59 x, r = 0.571, p = 0.0015, n = 28) between ATP-dependent Ca2+ uptake and gestational age (Fig. 7).
ATP-dependent Ca2+ uptake in BM vesicles isolated from placentas during the last trimester of human pregnancy. The reaction mixture was incubated for 10 min at 37°C with and without MgCl2-ATP. The ATP-dependent Ca2+ uptake increased linearly with gestational age (y = −168 + 5.59 x, r = 0.571, p = 0.0015, n = 28).
DISCUSSION
The main finding of the present study is that ATP-dependent Ca2+ uptake into human syncytiotrophoblast BM vesicles increased linearly during the third trimester. Our data suggest that this is due to an increased activity of the transporter rather than an increased protein expression. These changes are likely to be crucial in providing the necessary calcium for mineralization of the fetal bone mass in late gestation (17–20).
In an initial evaluation of the technique used to measure Ca2+ uptake, it was demonstrated that nonspecific binding to membranes was insignificant by using calcium-specific ionophore A23187. In addition, the ATP-dependent Ca2+ uptake was time dependent and linear during at least 10 min of incubation at 37°C and reached a plateau after 30 min of incubation. The inhibition experiment reported in this study together with previous reports (1, 2) suggests that most of the measured uptake is ATP dependent. Vanadate and strophanthidin inhibited more than 50% of the ATP-dependent calcium transport, implicating PMCA as the major transporter. This is supported by the absolute requirement of Mg2+ for the ATP-dependent transport. Consequently, these results together with the Western blot data suggest that ATP-dependent calcium transport across BM is mainly due to PMCA.
Our immunocytochemistry results correspond well to earlier findings of the distribution of the PMCA in the syncytiotrophoblast. Because the majority of the expression occurs in BM (3) and by using immunoblotting and isoform-specific antibodies against the PMCA, we have been able to demonstrate that isoforms 1 and 4 are present in the human syncytiotrophoblast BM. These results are in agreement with a previous study in which the mRNA for isoforms 1 and 4 were identified in human placenta (13). Isoforms 1 and 4 are both housekeeping isoforms found in many other tissues (12) and are suggested to be activated by different mechanisms. Isoform 1 is activated by cAMP and protein kinase A (PKA), whereas isoform 4 is activated by protein kinase C (PKC) and/or calmodulin (16). Isoform 2 is specific for brain and not found in placenta. Similarly, our results for isoform 3 indicate that it is not present in BM of syncytiotrophoblast.
Toward the end of pregnancy, requirements of the human fetus for calcium increase due to the accelerating rate of bone mineralization. Using a mathematical model, Forbes (31) illustrated a decreased calcium accumulation rate based on a reduction in fetal growth rate after wk 35. Other studies have shown an increase in the rate of calcium accumulation in the last trimester from 60 mg of calcium per day up to 340 mg per day at the end of this trimester (19, 20). Furthermore, with new ultrasound methods, it has been shown that fetal growth continues to the end of gestation (32) rather than reaching a plateau. Consequently, transplacental transport of calcium must change in parallel to fetal growth and mineralization of the skeleton. In the rat, transplacental transport of Ca2+ increased 72-fold during the last days of gestation (10). In this species, it was demonstrated that placental PMCA mRNA expression was increased 2–3-fold during the same gestational period, whereas the mRNA levels of the calcium-binding calbindin 9k increased 135-fold. Glazier et al. (10) draw the conclusion that calbindin 9k is the rate limiting step in this ATP-dependent transport across the rat placenta. However, the PMCA must also maintain an increased transport of calcium to the fetus during this period of time. A 3-fold increase in mRNA expression suggests an increased PMCA expression that may contribute to the increased ATP-dependent Ca2+ transport. An activation of the existing pumps seems likely due to the large discrepancy in the changes in ATP-dependent Ca2+ transport and PMCA mRNA expression.
The necessary increase in maternal-fetal Ca2+ transfer in human pregnancy could be achieved by a rapid enlargement of the surface area of the fetal-facing plasma membrane of the syncytiotrophoblast, resulting in a marked increase in the total number of calcium pumps. However, this is unlikely to be the main mechanism because fetal growth is more rapid than the enlargement of BM surface area during this period of gestation (33, 34). In the current study, we addressed the possibility that PMCA protein expression is altered in the third trimester, which would allow for an increased transplacental transport. Our immunoblotting data showed an unchanged relative PMCA protein expression from 32 wk of gestation until term. However, the activity of the transporter increased linearly over the same gestational period. We suggest that this activation of the BM calcium pump is critical in supplying the rapidly growing fetus with sufficient amounts of Ca2+ for bone mineralization.
A multitude of regulatory mechanisms for PMCA have been described, and the stimulatory effect of calmodulin on pump activity is well established (35). Other activators of pump activity include acidic phospholipids and long-chain polyunsaturated fatty acids (36), PKA (16, 37, 38), PKC (15, 16), and the protease calpain (39). Whether any of these regulatory mechanisms are responsible for the observed gestational increase of Ca2+ ATPase activity in the human placental BM remains to be established. Furthermore, the existence of a fetal hormonal regulation of placental calcium pumps represents an interesting possibility and warrants further investigation. Although we found no change in BM PMCA expression in late gestation using an antibody recognizing both isoforms 1 and 4, it is possible that a change in the relative proportions of the two isoforms may contribute to the increased Ca2+ ATPase activity in the third trimester.
Abbreviations
- MVM:
-
microvillous membrane
- BM:
-
basal membrane
- PMCA:
-
plasma membrane Ca2+ ATPase
References
Fisher GJ, Kelley LK, Smith CH 1987 ATP-dependent calcium transport across basal plasma membranes of human placental trophoblast. Am J Physiol 252: C38–C46
Lafond J, Leclerc M, Brunette MG 1991 Characterisation of calcium transport by basal plasma membranes from human placental syncytiotrophoblast. J Cell Physiol 148: 17–23
Borke JL, Caride AK, Verma AK, Kelley LK, Smith CH, Penniston JT, Kumar R 1989 Calcium pump epitopes in placental trophoblast plasma membranes. Am J Physiol 257: C341–C346
Borke JL, Minami J, Verma AK, Penniston JT, Kumar R 1988 Co-localization of erythrocyte Ca++-Mg++ ATPase and vitamin D-dependent 28 kilodalton calcium binding protein in cells of human kidney distal tubules. Kidney Int 34: 262–267
Borke JT, Caride A, Verma AK, Penniston JT, Kumar R 1990 Cellular and segmental distribution of Ca++ pump epitopes in rat intestine. Pflugers Arch 417: 120–122
Kikuchi K, Kikuchi T, Ghisham FK 1988 Characterization of calcium transport by basolateral membrane vesicles of human small intestine. Am J Physiol 255: G482–G489
Kamath SG, Kelley LK, Friedman AF, Smith CH 1992 Transport and binding in calcium uptake by microvillous membrane of human placenta. Am J Physiol 262: C789–C794
Johnson JA, Kumar R 1994 Renal and intestinal calcium transport: roles of vitamin D-dependent calcium binding proteins. Semin Nephrol 14: 119–128
Hosking DJ 1996 Calcium homeostasis in pregnancy. Clin Endocrinol 45: 1–6
Glazier JD, Atikson DE, Thornburgh KL, Sharpe PT, Edward D, Boyd RDH, Sibley CP 1992 Gestational changes in Ca2+ transport across rat placenta and mRNA for calbindin 9K and Ca2+ ATPase. Am J Physiol 262: R930–R935
Brun P, Dupret JM, Perret C, Thomasset M, Mathieu H 1987 Vitamin D-dependent calcium-binding proteins (CaBPs) in human fetuses: comparative distribution of 9K CaBP mRNA and 28K CaBP during development. Pediatr Res 21: 362–367
Stauffer TP, Guerini D, Carafoli E 1995 Tissue distribution of the four gene products of the plasma membrane Ca2+ pump. J Biol Chem 270: 12184–12190
Howard A, Legon S, Walters JRF 1992 Plasma membrane calcium pump expression in human placenta and small intestine. Biochim Biophys Res Commun 183: 499–505
Glazier JD, Ayuk P, Grey A, Sides K 1998 Syncytiotrophoblast basal membrane isolation. Placenta 19: 443–444
Zylinska L, Guerini D, Gromadzinska E, Lachowicz L 1998 Protein kinases A and C phosphorylate purified Ca2+-ATPase from rat cortex, cerebellum, and hippocampus. Biochim Biophys Acta 1448: 99–108
Verma AK, Patszty K, Filoteo AG, Penniston JT, Enyedi A 1999 Protein kinase C phosphorylates plasma membrane Ca2+ pump isoform 4a at its calmodulin binding domain. J Biol Chem 275: 527–523
Ryan S, Conngdom PJ, James J, Truscott J, Horsman A 1988 Mineral accretion in the human fetus. Arch Dis Child 63: 799–808
Minton SD, Steichen JJ, Tsang RC 1979 Bone mineral content in term and preterm appropriate-for-gestational-age-infants. J Pediatr 95: 1037–1042
Ziegler EE, O'Donnell AM, Nelson SE, Fomon SJ 1976 Body composition of reference fetus. Growth 40: 329–341
Shaw JCL 1976 Evidence for defective skeletal mineralization in low-birthweight infants: the absorption of calcium and fat. Pediatrics 57: 16–25
Illsley NP, Wang ZQ, Gray A, Sellers MC, Jacobs MM 1990 Simultaneous preparation of paired, syncytial, microvillous and basal membranes from human placenta. Biochim Biophys Acta 1029: 218–222
Schultz G, Jakbos KH 1984 Adenylate cyclase. In: Bergmeyer H (ed) Methods of Enzymatic Analysis. Verlag Chemie, Weinham, Germany, pp 369–378
Bowers GN, McComb RB 1966 A continuous spectrophotometric method for measuring the activity of serum alkaline phosphates. Clin Chem 12: 70–89
Ives HE, Casnellie JE, Greengard P, Jamieson JD 1980 Subcellular localization of cyclic GMP-dependent protein kinase and its substrates in vascular smooth muscle. J Biol Chem 255: 3777–3785
Bonner WD 1955 Succinic dehydrogenase. In: Colowick SP, Kalpan NO (eds) Methods in Enzymology, Vol I. Academic Press, New York, pp 722–729
Bradford MM 1976 A rapid and sensitive method for the quantitation of micrograms of quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254
Johansson M, Jansson T, Powell TL 2000 Na+/K+-ATPase is distributed to the microvillous and basal membrane of the syncytiotrophoblast in human placenta. Am J Physiol (in press)
Schatzmann HJ, Bürgin H 1978 Calcium in human red blood cells. Ann N Y Acad Sci 307: 124–147
Di Polo R, Rojas HR, Beauge L 1979 Vanadate inhibits uncoupled Ca efflux but not Na-Ca exchange in squid axons. Nature 281: 228–229
Xu K 1992 Inhibition of H+-transporting ATPase, Ca2+-transporting ATPase, and H+/K+-transporting ATPase by strophanthidin. Biochim Biophys Acta 1159: 109–112
Forbes GB 1988 Some remarks on bone mineralizations. J Pediatr 113: 167–171
Marsal K, Persson PH, Larsen H, Selbing A, Sultan B 1996 Intrauterine growth curves based on ultrasonically estimated foetal weights. Acta Pædiatr 85: 843–848
Baur R 1977 Morphometry of the placental exchange area. In: Advances in Anatomy, Embryology, and Cell Biology, Vol 53. Springer-Verlag, Berlin, p 5–65
Teasdale F 1980 Gestational changes in the functional structure of the human placenta in relation to fetal growth: a morphometric study. Am J Obstet Gynecol 137: 560–568
Jarrett HW, Penniston JT 1977 Partial purification of the (Ca2+ Mg2+) ATPase activator from human erythrocytes: its similarity to the activator of 3′-5′-cyclic nucleotide phosphodiesterase. Biochim Biophys Res Commun 77: 1210–1216
Ronner P, Gazzotti P, Carafoli E 1977 A lipid requirement for the (Ca2+ + Mg2+)- activated ATPase of erythrocyte membranes. Arch Biochem Biophys 179: 578–583
James PH, Pruschy M, Vorherr T, Penniston JT, Carafoli E 1989 Primary structure of the cAMP-dependent phosphorylation site of the plasma membrane calcium pump. Biochemistry 28: 4253–4258
Carafoli E 1994 Biogenesis: plasma membrane calcium ATPase: 15 years of work on the purified enzyme. FASEB J 8: 993–1002
Molinari M, Carafoli E 1997 Calpain: a cytosolic proteinase active at the membranes. J Membr Biol 156: 1–8
Acknowledgements
The authors thank Elisabet Pollak for her assistance with the plasma membrane isolation.
Author information
Authors and Affiliations
Additional information
Supported by Swedish Medical Research Council (10838, 11834), the Emil and Vera Cornell Foundation, Frimurare-Barnhus-Direktionen, the Åhlens Foundation, the General Maternity Hospital Foundation, the Crafoord Foundation, the Willhelm and Martina Lundgrens Foundation, the Kungliga och Hvitfeldtska Foundation, and the Samariten Foundation.
Rights and permissions
About this article
Cite this article
Strid, H., Powell, T. ATP-Dependent Ca2+ Transport Is Up-Regulated during Third Trimester in Human Syncytiotrophoblast Basal Membranes. Pediatr Res 48, 58–63 (2000). https://doi.org/10.1203/00006450-200007000-00012
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-200007000-00012
This article is cited by
-
Phosphate, calcium, and vitamin D signaling, transport, and metabolism in the endometria of cyclic ewes
Journal of Animal Science and Biotechnology (2023)
-
Activity and expression of Na+/H+ exchanger isoforms in the syncytiotrophoblast of the human placenta
Pflügers Archiv - European Journal of Physiology (2005)